Systematic Investigation of Silicon Content Effects on the PEO Coatings’ Properties on Al–Si Binary Alloys in Silicate-Based and Tungstate-Containing Electrolytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
2.2. Plasma Electrolytic Oxidation (PEO) Process
2.3. Coating Characterization
2.4. Micro-Hardness and Wear Measurement
3. Results and Discussion
3.1. Microstructure and Micro-Hardness of the Substrates
3.2. Voltage Variation during PEO Treatment
3.3. The Characterization and Analysis of Coatings
3.3.1. Coatings’ Surface Morphology and Chemical Composition
3.3.2. Characterization of Cross-Section
3.3.3. The Surface Roughness and Thickness of the Coatings
3.3.4. The Phase Analysis
3.4. Wear Behavior
4. Conclusions
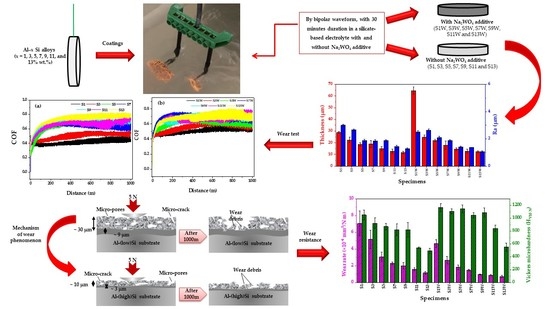
- The bright areas on the surface of the high-Si specimens were mainly composed of silicon oxides. The change in the surface micrographs of the coatings formed in the additive-containing electrolyte is negligible. Furthermore, the effect of the substrate surface’s primary structure was not recognizable for both coatings with and without additives.
- The highest and lowest porosity percentages were measured for the S13 (i.e., 27.46%) and the S1W coating (i.e., 6.68%), respectively. The finer sparking in the electrolyte containing Na2WO4 led to the lower porosity in all of the obtained coatings compared with the coatings produced without the additive electrolyte. In addition, the porosity percent was incremented with the increasing silicon content of the substrate.
- The mean thickness of the coatings was increased by the presence of Na2WO4 (S1W~64.6 μm) in the electrolyte. On the other hand, increasing the Si content of the substrate for the specimens both with and without the additive decreased the average thickness.
- The lower surface roughness was attributed to the coating formed in the additive-containing electrolyte due to its higher conductivity of 21.8 mS cm−1. However, by further increasing the silicon content of the substrate, the coatings became smoother.
- The higher wear rates, volume losses, track widths, and depths were related to the specimens with a lower silicon content of the substrate due to the greater outer layer thicknesses of the coatings. However, the coating produced using the additive-containing electrolyte showed a higher micro-hardness and a lower wear rate in comparison with the coatings produced in the silicate-based electrolyte.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Nie, X.; Wang, L.; Northwood, D.O. Corrosion protection properties of anodic oxide coatings on an Al-Si alloy. Surf. Coat. Technol. 2005, 200, 1994–2000. [Google Scholar] [CrossRef]
- Shirani, A.; Joy, T.; Rogov, A.; Lin, M.; Yerokhin, A.; Mogonye, J.E.; Korenyi-Both, A.; Aouadi, S.M.; Voevodin, A.A.; Berman, D. PEO-Chameleon as a potential protective coating on cast aluminum alloys for high-temperature applications. Surf. Coat. Technol. 2020, 397, 126016. [Google Scholar] [CrossRef]
- Xu, F.; Xia, Y.; Li, G. The mechanism of PEO process on Al-Si alloys with the bulk primary silicon. Appl. Surf. Sci. 2009, 255, 9531–9538. [Google Scholar] [CrossRef]
- He, J.; Cai, Q.Z.; Luo, H.H.; Yu, L.; Wei, B.K. Influence of silicon on growth process of plasma electrolytic oxidation coating on Al-Si alloy. J. Alloys Compd. 2009, 471, 395–399. [Google Scholar] [CrossRef]
- Zhu, B.; Seifeddine, S.; Persson, P.O.Å.; Jarfors, A.E.W.; Leisner, P.; Zanella, C. A study of formation and growth of the anodised surface layer on cast Al-Si alloys based on different analytical techniques. Mater. Des. 2016, 101, 254–262. [Google Scholar] [CrossRef]
- Li, K.; Li, W.; Zhang, G.; Zhu, W.; Zheng, F.; Zhang, D. Effects of Si phase refinement on the plasma electrolytic oxidation of eutectic Al-Si alloy. J. Alloys Compd. 2019, 790, 650–656. [Google Scholar] [CrossRef]
- Mora-Sanchez, H.; del Olmo, R.; Rams, J.; Torres, B.; Mohedano, M.; Matykina, E.; Arrabal, R. Hard Anodizing and Plasma Electrolytic Oxidation of an Additively Manufactured Al-Si alloy. Surf. Coat. Technol. 2021, 420, 127339. [Google Scholar] [CrossRef]
- Mistry, K.; Priest, M.; Shrestha, S. The potential of plasma electrolytic oxidized eutectic aluminium-silicon alloy as a cylinder wall surface for lightweight engine blocks. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2010, 224, 221–229. [Google Scholar] [CrossRef]
- Barati Darband, G.; Aliofkhazraei, M.; Hamghalam, P.; Valizade, N. Plasma electrolytic oxidation of magnesium and its alloys: Mechanism, properties and applications. J. Magnes. Alloy. 2017, 5, 74–132. [Google Scholar] [CrossRef]
- Tjiang, F.; Ye, L.W.; Huang, Y.J.; Chou, C.C.; Tsai, D.S. Effect of processing parameters on soft regime behavior of plasma electrolytic oxidation of magnesium. Ceram. Int. 2017, 43, S567–S572. [Google Scholar] [CrossRef]
- Lu, X.; Mohedano, M.; Blawert, C.; Matykina, E.; Arrabal, R.; Kainer, K.U.; Zheludkevich, M.L. Plasma electrolytic oxidation coatings with particle additions—A review. Surf. Coat. Technol. 2016, 307, 1165–1182. [Google Scholar] [CrossRef]
- Wang, L.; Nie, X. Silicon effects on formation of EPO oxide coatings on aluminum alloys. Thin Solid Films 2006, 494, 211–218. [Google Scholar] [CrossRef]
- Alves, S.A.; Fern, P.; Bay, R. Enhanced tribological performance of cylinder liners made of cast aluminum alloy with high silicon content through plasma electrolytic oxidation. Surf. Coat. Technol. 2022, 433, 128146. [Google Scholar] [CrossRef]
- Polunin, A.V.; Cheretaeva, A.O.; Borgardt, E.D.; Rastegaev, I.A.; Krishtal, M.; Katsman, A.V.; Yasnikov, I.S. Improvement of oxide layers formed by plasma electrolytic oxidation on cast Al–Si alloy by incorporating TiC nanoparticles. Surf. Coat. Technol. 2021, 423, 127603. [Google Scholar] [CrossRef]
- Wen, L.; Wang, Y.; Zhou, Y.; Ouyang, J.; Guo, L.; Jia, D. Corrosion evaluation of microarc oxidation coatings formed on 2024 aluminium alloy. Corros. Sci. 2010, 52, 2687–2696. [Google Scholar] [CrossRef]
- Olmo, R.; Mohedano, M.; Visser, P.; Matykina, E.; Arrabal, R. Flash-PEO coatings loaded with corrosion inhibitors on AA2024. Surf. Coat. Technol. 2020, 402, 126317. [Google Scholar] [CrossRef]
- Yan, H.; Liu, W.; Yu, Z.; Liu, B.; Liu, C. Effect of Sodium Tungstate on the Microstructure and Properties of Micro-Arc Oxidized Coatings Formed on 2A12 Aluminum Alloy. J. Mater. Eng. Perform. 2021, 30, 7741–7751. [Google Scholar] [CrossRef]
- Tseng, C.C.; Lee, J.L.; Kuo, T.H.; Kuo, S.N.; Tseng, K.H. The influence of sodium tungstate concentration and anodizing conditions on microarc oxidation (MAO) coatings for aluminum alloy. Surf. Coat. Technol. 2012, 206, 3437–3443. [Google Scholar] [CrossRef]
- Toulabifard, A.; Hakimizad, A.; Di Franco, F.; Raeissi, K.; Santamaria, M. Synergistic effect of W incorporation and pulsed current mode on wear and tribocorrosion resistance of coatings grown by plasma electrolytic oxidation on 7075 Al alloy. Mater. Res. Express. 2019, 6, 106502. [Google Scholar] [CrossRef]
- Hakimizad, A.; Raeissi, K.; Santamaria, M.; Asghari, M. Effects of pulse current mode on plasma electrolytic oxidation of 7075 Al in Na2WO4 containing solution: From unipolar to soft-sparking regime. Electrochim. Acta J. 2018, 164, C690–C698. [Google Scholar] [CrossRef]
- Vander Voort, G.F.; Asensio-lozano, J. The Al-Si Phase Diagram. Microsc. Microanal. 2009, 15, 60–61. [Google Scholar] [CrossRef]
- Uguz, A.; Bayram, A. Effect of Si content and microstructure on the wear behaviour of Al-Si alloys. Metall 2001, 55, 758–761. [Google Scholar]
- Dahle, A.K.; Nogita, K.; McDonald, S.D.; Dinnis, C.; Lu, L. Eutectic modification and microstructure development in Al-Si Alloys. Mater. Sci. Eng. A 2005, 413–414, 243–248. [Google Scholar] [CrossRef]
- Zheng, H.Y.; Wang, Y.K.; Li, B.S.; Han, G.R. The effects of Na2WO4 concentration on the properties of microarc oxidation coatings on aluminum alloy. Mater. Lett. 2005, 59, 139–142. [Google Scholar] [CrossRef]
- Di Chen, X.; Cai, Q.Z.; Yin, L.S. Effects of Na2WO4 Additive on Properties of Plasma Electrolytic Oxidation Coatings on 6061 Al Alloy. Adv. Chem. Eng. II 2012, 550–553, 1969–1975. [Google Scholar] [CrossRef]
- Hussein, R.O.; Nie, X.; Northwood, D.O. An investigation of ceramic coating growth mechanisms in plasma electrolytic oxidation (PEO) processing. Electrochim. Acta 2013, 112, 111–119. [Google Scholar] [CrossRef]
- Rogov, A.B.; Yerokhina, A.; Matthewsa, A. The role of cathodic current in plasma electrolytic oxidation of aluminum: Phenomenological concepts of the “soft sparking” mode. Prepr. Langmuir. 2017, 33, 11059–11069. [Google Scholar] [CrossRef]
- Tillous, K.; Toll-Duchanoy, T.; Bauer-Grosse, E.; Hericher, L.; Geandier, G. Microstructure and phase composition of microarc oxidation surface layers formed on aluminium and its alloys 2214-T6 and 7050-T74. Surf. Coat. Technol. 2009, 203, 2969–2973. [Google Scholar] [CrossRef]
- Wang, P.; Li, J.P.; Guo, Y.C.; Yang, Z.; Wang, J.L. Ceramic coating formation on high Si containing Al alloy by PEO process. Surf. Eng. 2016, 32, 428–434. [Google Scholar] [CrossRef]
- Li, K.; Yao, W.; Xie, Y.; Zhang, J.; Li, B.; Wan, Z.; Zhang, Z.; Lu, L.; Tang, Y. AC plasma electrolytic oxidation of additively manufactured and cast AlSi12 alloys. Surf. Coat. Technol. 2020, 399, 125708. [Google Scholar] [CrossRef]
- Terleeva1, O.P.; Oh, Y.; Slonova, A.I.; Kireenko, I.B.; Ok, M.-R.; Ha, H.-P. Quantitative parameters and definition of stages of anodic-cathodic microplasma processes on aluminum alloys. Mater. Trans. 2005, 46, 2077–2082. [Google Scholar] [CrossRef]
- Sah, S.P.; Tsuji, E.; Aoki, Y.; Habazaki, H. Cathodic pulse breakdown of anodic films on aluminium in alkaline silicate electrolyte—Understanding the role of cathodic half-cycle in AC plasma electrolytic oxidation. Corros. Sci. 2012, 55, 90–96. [Google Scholar] [CrossRef]
- Duan, H.; Yan, C.; Wang, F. Growth process of plasma electrolytic oxidation films formed on magnesium alloy AZ91D in silicate solution. Electrochim. Acta 2007, 52, 5002–5009. [Google Scholar] [CrossRef]
- Chang, L. Growth regularity of ceramic coating on magnesium alloy by plasma electrolytic oxidation. J. Alloys Compd. 2009, 468, 462–465. [Google Scholar] [CrossRef]
- Li, K.; Zhang, G.; Yi, A.; Zhu, W.; Liao, Z.; Chen, K.; Li, W.; Luo, Z. Effects of Matrix Silicon Content on the Plasma Electrolytic Oxidation of Al-Si Alloys Using Different Power Modes. Crystals 2022, 12, 123. [Google Scholar] [CrossRef]
- Clyne, T.W.; Troughton, S.C. A review of recent work on discharge characteristics during plasma electrolytic oxidation of various metals. Int. Mater. Rev. 2019, 64, 127–162. [Google Scholar] [CrossRef]
- Madhan Kumar, A.; Kwon, S.H.; Jung, H.C.; Shin, K.S. Corrosion protection performance of single and dual Plasma Electrolytic Oxidation (PEO) coating for aerospace applications. Mater. Chem. Phys. 2015, 149, 480–486. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Xue, Z.G.; Wang, Q.; Wu, X.Q.; Matykina, E.; Skeldon, P.; Thompson, G.E. New findings on properties of plasma electrolytic oxidation Coatings from study of an Al-Cu-Li alloy. Electrochim. Acta 2013, 107, 358–378. [Google Scholar] [CrossRef]
- Hussein, R.O.; Northwood, D.O.; Su, J.F.; Nie, X. A study of the interactive effects of hybrid current modes on the tribological properties of a PEO (plasma electrolytic oxidation) coated AM60B Mg-alloy. Surf. Coat. Technol. 2013, 215, 421–430. [Google Scholar] [CrossRef]
- Xiang, N.; Song, R.G.; Wang, C.; Mao, Q.Z.; Ge, Y.J.; Ding, J.H.; Song, R.G.; Wang, C.; Mao, Q.Z.; Ge, Y.J.; et al. Formation of corrosion resistant plasma electrolytic oxidation coatings on aluminium alloy with addition of sodium tungstate species. Corros. Eng. Sci. Technol. 2016, 51, 146–154. [Google Scholar] [CrossRef]
- Dai, L.; Li, W.; Zhang, G.; Fu, N.; Duan, Q. Anti-corrosion and wear properties of plasma electrolytic oxidation coating formed on high Si content Al alloy by sectionalized oxidation mode. J. Phys. Conf. Ser. Mater. Sci. Eng. 2016, 167, 012063. [Google Scholar] [CrossRef]
- Hashemzadeh, M.; Raeissi, K.; Ashrafizadeh, F.; Hakimizad, A.; Santamaria, M.; Lampke, T. Silicate and Hydroxide Concentration Influencing the Properties of Composite Al2O3-TiO2 PEO Coatings on AA7075 Alloy. Coatings 2022, 12, 33. [Google Scholar] [CrossRef]
- Hussein, R.O.; Nie, X.; Northwood, D.O.; Yerokhin, A.; Matthews, A. Spectroscopic study of electrolytic plasma and discharging behaviour during the plasma electrolytic oxidation (PEO) process. J. Phys. D Appl. Phys. 2010, 43, 105–203. [Google Scholar] [CrossRef]
- Hussein, R.O.; Zhang, P.; Nie, X.; Xia, Y.; Northwood, D.O. The effect of current mode and discharge type on the corrosion resistance of plasma electrolytic oxidation (PEO) coated magnesium alloy AJ62. Surf. Coat. Technol. 2011, 206, 1990–1997. [Google Scholar] [CrossRef]
- Hussein, R.O.; Northwood, D.O. Improving the performance of magnesium alloys for automotive applications. WIT Trans. Built Environ. 2014, 137, 531–544. [Google Scholar] [CrossRef]
- Blawert, C.; Srinivasan, P.B. 6—Plasma electrolytic oxidation treatment of magnesium alloys. In Surface Engineering Light Alloy; Dong, H., Ed.; Woodhead Publishing: Sawston, UK; GKSS-Forschungszentrum Geesthacht GmbH: Geesthacht, Germany, 2010; Volume 1, pp. 155–183. [Google Scholar] [CrossRef]
- Jiang, B.L.; Wang, Y.M. Plasma electrolytic oxidation treatment of aluminium and titanium alloys. In Surface Engineering Light Alloy; Dong, H., Ed.; Woodhead Publishing: Sawston, UK; Xi’an University of Technology: Xi’an, China, 2010; Volume 1, pp. 110–154. [Google Scholar]
- Hussein, R.O.; Northwood, D.O.; Nie, X. Processing-Microstructure Relationships in the Plasma Electrolytic Oxidation (PEO) Coating of a Magnesium Alloy. Mater. Sci. Appl. 2014, 5, 124–139. [Google Scholar] [CrossRef]
- Lu, X.; Blawert, C.; Scharnagl, N.; Kainer, K.U. Influence of incorporating Si3N4 particles into the oxide layer produced by plasma electrolytic oxidation on AM50 Mg alloy on coating morphology and corrosion properties. J. Magnes. Alloy. 2013, 1, 267–274. [Google Scholar] [CrossRef]
- Hwang, I.J.; Shin, K.R.; Lee, J.S.; Ko, Y.G.; Shin, D.H. Formation of Black Ceramic Layer on Aluminum Alloy by Plasma Electrolytic Oxidation in Electrolyte Containing Na2WO4. Mater. Trans. 2012, 53, 559–564. [Google Scholar] [CrossRef]
- Stojadinovic, S.; Vasilic, R.; Belca, I.; Petkovic, M.; Kasalica, B.; Nedic, Z.; Zekovic, L. Characterization of the plasma electrolytic oxidation of aluminium in sodium tungstate. Corros. Sci. 2010, 52, 3258–3265. [Google Scholar] [CrossRef]
- Li, H.; Yin, Z.; Deng, L.; Wang, S.; Fu, Z.; Ma, Y. Effect of SiO2/Al2O3 ratio on the structure and electrical properties of MgO–Al2O3–SiO2 glass-ceramics doped with TiO2. Mater. Chem. Phys. 2020, 256, 3–9. [Google Scholar] [CrossRef]
- Hakimizad, A.; Raeissi, K.; Golozar, M.A.; Lu, X.; Blawert, C.; Zheludkevich, M.L. Influence of cathodic duty cycle on the properties of tungsten containing Al2O3/TiO2 PEO nano-composite coatings. Surf. Coat. Technol. 2018, 340, 210–221. [Google Scholar] [CrossRef]
- Forn, A.; Picas, J.A.; Baile, M.T.; Martin, E.; García, V.G. Microstructure and tribological properties of anodic oxide layer formed on Al-Si alloy produced by semisolid processing. Surf. Coat. Technol. 2007, 202, 1139–1143. [Google Scholar] [CrossRef]
- Malayoglu, U.; Tekin, K.C.; Malayoglu, U.; Shrestha, S. An investigation into the mechanical and tribological properties of plasma electrolytic oxidation and hard-anodized coatings on 6082 aluminum alloy. Mater. Sci. Eng. A 2011, 528, 7451–7460. [Google Scholar] [CrossRef]
- Durdu, S.; Bayramoglu, S.; Demirtaş, A.; Usta, M.; Üçşk, A.H. Characterization of AZ31 Mg Alloy coated by plasma electrolytic oxidation. Vacuum 2013, 88, 130–133. [Google Scholar] [CrossRef]
- Evangeline, A.; Sathiya, P.; Prabu, S.S.; Devendranath, K.; Arivazhagan, N. Effect of Coating Thickness on Wear Performance of Inconel 625 coating. IOP Conf. Ser. Mater. Sci. Eng. Pap. 2018, 423, 012159. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, F.; Zhang, Y.; Du, C. Influence of graphene oxide additive on the tribological and electrochemical corrosion properties of a PEO coating prepared on AZ31 magnesium alloy. Tribol. Int. 2020, 146, 106135. [Google Scholar] [CrossRef]
- Arrabal, R.; Mohedano, M.; Mingo, B.; Matykina, E.; Pardo, A.; Merino, M.C. Characterization and wear behaviour of PEO coatings on 6082-T6 aluminum alloy with incorporated α-Al2O3 particles. Surf. Coat. Technol. 2015, 269, 64–73. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, L.; Qi, Y.; Cai, W.; Jiang, Z. Self-lubricating Al2O3/PTFE composite coating formation on surface of aluminium alloy. Surf. Coat. Technol. 2010, 204, 3315–3318. [Google Scholar] [CrossRef]
- Rahmati, M.; Raeissi, K.; Toroghinejad, M.R.; Hakimizad, A.; Santamaria, M. Corrosion and wear resistance of coatings produced on AZ31 Mg alloy by plasma electrolytic oxidation in silicate-based K2TiF6 containing solution: Effect of waveform. J. Magnes. Alloy. 2021. [Google Scholar] [CrossRef]
- Li, Z.; Di, S. Preparation and properties of microarc oxidation self-lubricating composite coatings on aluminum alloy. Metals 2017, 7, 127. [Google Scholar] [CrossRef] [Green Version]



















| Specimen | Element (wt.%) | Stable Phase | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Al | Si | Fe | Cu | Mn | Mg | Zn | Cr | ||
| -Al–1%Si | 98.80 | 1.02 | 0.13 | 0.001 | 0.041 | 0.0006 | 0.006 | 0.002 | α + fine eutectic (α + β) phase |
| Al–3%Si | 96.85 | 2.93 | 0.16 | 0.023 | 0.017 | 0.0001 | 0.007 | 0.008 | α + eutectic (α + β) phase |
| Al–5%Si | 95.07 | 4.83 | 0.17 | 0.034 | 0.032 | 0.0002 | 0.011 | 0.005 | α + eutectic (α + β) phase |
| Al–7%Si | 92.81 | 6.87 | 0.19 | 0.058 | 0.042 | 0.0005 | 0.017 | 0.006 | α + eutectic (α + β) phase |
| Al–9%Si | 90.51 | 9.11 | 0.23 | 0.075 | 0.055 | 0.0001 | 0.015 | 0.007 | α + eutectic (α + β) phase |
| Al–11%Si | 88.45 | 11.10 | 0.26 | 0.095 | 0.068 | 0.0002 | 0.023 | 0.007 | β + eutectic (α + β) phase |
| Al–13%Si | 86.66 | 12.87 | 0.27 | 0.096 | 0.069 | 0.0002 | 0.027 | 0.007 | β + eutectic (α + β) phase |
| Specimen Code | Wear Rate (×10−4 mm3/N m) | Worn Volume (mm3) | Track Width (mm) | Max. Depth (µm) | COF | Track Depth/Thickness of Coatings |
|---|---|---|---|---|---|---|
| S1 | 7.05 ± 1.5 | 3.52 ± 0.76 | 2.34 ± 0.27 | 20.8 ± 3.1 | 0.45–0.5 | 0.72 |
| S3 | 5.15 ± 1.1 | 2.58 ± 0.53 | 2.25 ± 0.30 | 16.12 ± 4.2 | 0.51–0.56 | 0.71 |
| S5 | 3.04 ± 0.57 | 1.52 ± 0.28 | 2.04 ± 0.06 | 14.31 ± 4.7 | 0.65–0.7 | 0.76 |
| S7 | 2.29 ± 0.16 | 1.15 ± 0.08 | 1.72 ± 0.14 | 12.87 ± 0.85 | 0.58–0.7 | 0.68 |
| S9 | 1.99 ± 0.38 | 0.99 ± 0.18 | 1.68 ± 0.30 | 9.79 ± 2.19 | 0.68–0.71 | 0.65 |
| S11 | 1.58 ± 0.17 | 0.79 ± 0.08 | 1.14 ± 0.14 | 7.67 ± 2.9 | 0.72–0.79 | 0.59 |
| S13 | 1.14 ± 0.19 | 0.57 ± 0.00 | 1.12 ± 0.01 | 7.09 ± 0.77 | 0.76–0.81 | 0.61 |
| S1W | 4.66 ± 0.47 | 2.33 ± 0.24 | 1.36 ± 0.05 | 19.95 ± 3.46 | 0.5–0.58 | 0.31 |
| S3W | 2.66 ± 0.45 | 1.33 ± 0.23 | 1.45 ± 0.14 | 16.08 ± 3.19 | 0.53–0.59 | 0.65 |
| S5W | 1.80 ± 0.26 | 0.90 ± 0.13 | 1.31 ± 0.26 | 12.90 ± 3.00 | 0.54–0.63 | 0.58 |
| S7W | 1.46 ± 0.11 | 0.73 ± 0.05 | 1.26 ± 0.25 | 11.74 ± 1.54 | 0.62–0.69 | 0.66 |
| S9W | 0.95 ± 0.06 | 0.47 ± 0.03 | 1.13 ± 0.35 | 9.91 ± 5.07 | 0.66–0.71 | 0.68 |
| S11W | 0.87 ± 0.08 | 0.44 ± 0.04 | 1.13 ± 0.18 | 7.17 ± 1.96 | 0.66–0.77 | 0.56 |
| S13W | 0.71 ± 0.13 | 0.35 ± 0.07 | 1.12 ± 0.20 | 6.71 ± 4.32 | 0.64–0.75 | 0.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moshrefifar, M.; Ebrahimifar, H.; Hakimizad, A. Systematic Investigation of Silicon Content Effects on the PEO Coatings’ Properties on Al–Si Binary Alloys in Silicate-Based and Tungstate-Containing Electrolytes. Coatings 2022, 12, 1438. https://doi.org/10.3390/coatings12101438
Moshrefifar M, Ebrahimifar H, Hakimizad A. Systematic Investigation of Silicon Content Effects on the PEO Coatings’ Properties on Al–Si Binary Alloys in Silicate-Based and Tungstate-Containing Electrolytes. Coatings. 2022; 12(10):1438. https://doi.org/10.3390/coatings12101438
Chicago/Turabian StyleMoshrefifar, Masoud, Hadi Ebrahimifar, and Amin Hakimizad. 2022. "Systematic Investigation of Silicon Content Effects on the PEO Coatings’ Properties on Al–Si Binary Alloys in Silicate-Based and Tungstate-Containing Electrolytes" Coatings 12, no. 10: 1438. https://doi.org/10.3390/coatings12101438
APA StyleMoshrefifar, M., Ebrahimifar, H., & Hakimizad, A. (2022). Systematic Investigation of Silicon Content Effects on the PEO Coatings’ Properties on Al–Si Binary Alloys in Silicate-Based and Tungstate-Containing Electrolytes. Coatings, 12(10), 1438. https://doi.org/10.3390/coatings12101438