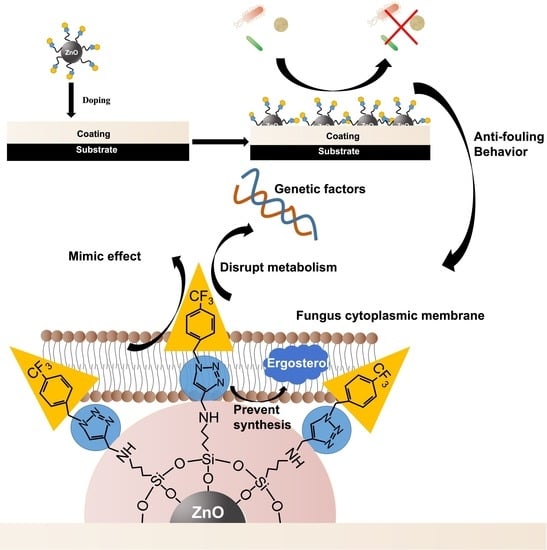
Fluorinated-Triazole-Modified ZnO and Its Application in Marine Antifouling
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Methods
2.2.1. Preparation of ZnO−APTES
2.2.2. Preparation of ZnO−APTES−F
2.2.3. Preparation of ZnO−APTES−TRF
2.2.4. Preparation of Composite Coatings
2.3. Characterization
2.3.1. Characterization of ZnO−APTES, ZnO−APTES−F and ZnO−APTES−TRF
2.3.2. Antifouling Property Tests
3. Results and Discussion
3.1. Characterization of ZnO−APTES, ZnO−APTES−F, and ZnO−APTES−TRF
3.1.1. Microstructure Characterization
3.1.2. Surface Analysis
3.1.3. Graft Analysis
3.2. Antibacterial Properties of ZnO−APTES−TRF
3.3. Antibacterial Property Test of Coating
3.4. Marine Environment Test
4. Conclusions
- (1)
- The antibacterial efficiency of fluorinated aromatic hydrocarbons and triazole rings was studied by antibacterial laboratory tests, which showed that fluorinated aromatic hydrocarbons enhanced the antibacterial efficiency of ZnO, and the presence of the triazole ring group greatly enhanced the antibacterial activity of the nano−ZnO. The inhibition rate of the ZnO−APTES−TRF on Escherichia coli, Staphylococcus aureus, and Pseudoalteromonas sp. can reach more than 98%. The ZnO−APTES−TRF/ZA coating can keep the antifouling effect in the natural marine environment for more than 120 days and has good long−term use.
- (2)
- The rate of grafting of triazole ring fluorinated aromatic hydrocarbons on the surface of the ZnO−APTES−TRF can reach 32.38%. Through verification, the weight ratio of triazole ring to fluoroaromatic hydrocarbon is close to the theoretical value of 1:2, which is in line with the expectation. When coated with the ZA resin, the water contact angle of the ZnO−APTES−TRF/ZA surface can reach 106°, indicating a hydrophobic surface.
- (3)
- Due to the wide application of triazole ring groups and fluorinated aromatic hydrocarbons in biomedicine, the ZnO−APTES−TRF with a low toxicity and high safety has potential application value in marine fisheries, aquaculture, shipping, and other industries.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, L.; Duan, Y.; Cui, M.; Huang, R.; Su, R.; Qi, W.; He, Z. Biomimetic surface coatings for marine antifouling: Natural antifoulants, synthetic polymers and surface microtopography. Sci. Total Environ. 2021, 766, 144469. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Tian, L.; Bing, W.; Zhao, J.; Ren, L. Bioinspired marine antifouling coatings: Status, prospects, and future. Prog. Mater. Sci. 2022, 124, 100889. [Google Scholar] [CrossRef]
- Pourhashem, S.; Seif, A.; Saba, F.; Nezhad, E.G.; Ji, X.; Zhou, Z.; Zhai, X.; Mirzaee, M.; Duan, J.; Rashidi, A.; et al. Antifouling nanocomposite polymer coatings for marine applications: A review on experiments, mechanisms, and theoretical studies. J. Mater. Sci. Technol. 2022, 118, 73–113. [Google Scholar] [CrossRef]
- Bhoj, Y.; Tharmavaram, M.; Rawtani, D. A comprehensive approach to antifouling strategies in desalination, marine environment, and wastewater treatment. Chem. Phys. Impact 2021, 2, 100008. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, Y.; He, M.; Su, Y.; Zhao, X.; Elimelech, M.; Jiang, Z. Antifouling membranes for sustainable water purification: Strategies and mechanisms. Chem. Soc. Rev. 2016, 45, 5888–5924. [Google Scholar] [CrossRef] [PubMed]
- Satheesh, S.; Ba−akdah, M.A.; Al−Sofyani, A.A. Natural antifouling compound production by microbes associated with marine macroorganisms—A review. Electron. J. Biotechnol. 2016, 21, 26–35. [Google Scholar] [CrossRef] [Green Version]
- Tian, L.; Yin, Y.; Jin, H.; Bing, W.; Jin, E.; Zhao, J.; Ren, L. Novel marine antifouling coatings inspired by corals. Mater. Today Chem. 2020, 17, 100294. [Google Scholar] [CrossRef]
- Jin, H.; Wang, J.; Tian, L.; Gao, M.; Zhao, J.; Ren, L. Recent advances in emerging integrated antifouling and anticorrosion coatings. Mater. Des. 2022, 213, 110307. [Google Scholar] [CrossRef]
- Liu, M.; Li, S.; Wang, H.; Jiang, R.; Zhou, X. Research progress of environmentally friendly marine antifouling coatings. Polym. Chem. 2021, 12, 3702–3720. [Google Scholar] [CrossRef]
- Niu, B.; Chen, Y.; Zhang, L.; Tan, J. Organic–inorganic hybrid nanomaterials prepared via polymerization−induced self−assembly: Recent developments and future opportunities. Polym. Chem. 2022, 13, 2554–2569. [Google Scholar] [CrossRef]
- Simon, S.M.; George, G.; Sajna, M.S.; Prakashan, V.P.; Jose, T.A.; Vasudevan, P.; Saritha, A.C.; Biju, P.R.; Joseph, C.; Unnikrishnan, N.V. Recent advancements in multifunctional applications of sol−gel derived polymer incorporated TiO2−ZrO2 composite coatings: A comprehensive review. Appl. Surf. Sci. Adv. 2021, 6, 100173. [Google Scholar] [CrossRef]
- Gao, Y.; Yang, X.; Men, Y.; Wang, Y.; Xiang, Q.; Yue, F.; Ding, X.; Li, J.; Yang, Z.; Wang, Q. Superhydrophobic inorganic−organic composite coatings with hybrid micro−nano binary structure on copper. Polym. Adv. Technol. 2015, 26, 1320–1325. [Google Scholar] [CrossRef]
- Chen, J.−I.; Chareonsak, R.; Puengpipat, V.; Marturunkakul, S. Organic/Inorganic Composite Materials for Coating Applications. J. Appl. Polym. Sci. 1999, 74, 1341–1346. [Google Scholar] [CrossRef]
- Li, W.; Huang, D.; Xing, X.; Tang, J.; Xing, Y.; Li, X.; Zhang, J. Study the factors affecting the performance of organic−inorganic hybrid coatings. J. Appl. Polym. Sci. 2014, 131, 41010. [Google Scholar] [CrossRef]
- Luo, S.; Wei, J.; Xu, W.; Chen, Y.; Huang, H.; Hu, J.; Yu, Q. Design, preparation, and performance of a novel organic–inorganic composite coating with high adhesion and protection for concrete. Compos. Part B Eng. 2022, 234, 109695. [Google Scholar] [CrossRef]
- Ivanova, E.P.; Hasan, J.; Webb, H.K.; Gervinskas, G.; Juodkazis, S.; Truong, V.K.; Wu, A.H.; Lamb, R.N.; Baulin, V.A.; Watson, G.S.; et al. Bactericidal activity of black silicon. Nat. Commun. 2013, 4, 2838. [Google Scholar] [CrossRef]
- Sabzi, M.; Mirabedini, S.M.; Zohuriaan−Mehr, J.; Atai, M. Surface modification of TiO2 nano−particles with silane coupling agent and investigation of its effect on the properties of polyurethane composite coating. Prog. Org. Coat. 2009, 65, 222–228. [Google Scholar] [CrossRef]
- Ryu, J.Y.; Song, I.T.; Lau, K.H.; Messersmith, P.B.; Yoon, T.Y.; Lee, H. New antifouling platform characterized by single−molecule imaging. ACS Appl. Mater. Interfaces 2014, 6, 3553–3558. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Xie, C.; Ou, J.; Xue, M.; Wang, F.; Lei, S.; Fang, X.; Zhou, H.; Li, W. ZnO superhydrophobic coating via convenient spraying and its biofouling resistance. Surf. Interface Anal. 2018, 50, 1278–1285. [Google Scholar] [CrossRef]
- Dimitrakellis, P.; Ellinas, K.; Kaprou, G.D.; Mastellos, D.C.; Tserepi, A.; Gogolides, E. Bactericidal Action of Smooth and Plasma Micro-Nanotextured Polymeric Surfaces with Varying Wettability, Enhanced by Incorporation of a Biocidal Agent. Macromol. Mater. Eng. 2021, 306, 2000694. [Google Scholar] [CrossRef]
- Odds, F.C.; Brown, A.J.P.; Gow, N.A.R. Antifungal agents: Mechanisms of action. Trends Microbiol. 2003, 11, 272–279. [Google Scholar] [CrossRef]
- Karbasi, M.M.; Mirjafary, Z.; Saeidian, H.; Mokhtari, J. Efficient synthesis and DFT analysis of novel 1,2,3−triazole−based dithiocarbamates. J. Mol. Struct. 2021, 1227, 129535. [Google Scholar] [CrossRef]
- Cheng, R.; Yan, X.; Xu, Z. Isatin-1,2,3-triazole-isatin Scaffolds and Their Antibacterial Activity. J. Heterocycl. Chem. 2019, 56, 2970–2974. [Google Scholar] [CrossRef]
- Govindaiah, S.; Sreenivasa, S.; Ramakrishna, R.A.; Rao, T.M.C.; Nagabhushana, H. Regioselective Synthesis, Antibacterial, Molecular Docking and Fingerprint Applications of 1−Benzhydrylpiperazine Derivatized 1,4−Disubstituted 1,2,3−Triazoles. ChemistrySelect 2018, 3, 8111–8117. [Google Scholar] [CrossRef]
- Ouahrouch, A.; Ighachane, H.; Taourirte, M.; Engels, J.W.; Sedra, M.H.; Lazrek, H.B. Benzimidazole−1,2,3−triazole hybrid molecules: Synthesis and evaluation for antibacterial/antifungal activity. Arch. Pharm. 2014, 347, 748–755. [Google Scholar] [CrossRef] [Green Version]
- Rajabi−Moghaddam, H.; Naimi−Jamal, M.R.; Tajbakhsh, M. Fabrication of copper (II)−coated magnetic core−shell nanoparticles Fe3O4@SiO2−2−aminobenzohydrazide and investigation of its catalytic application in the synthesis of 1,2,3−triazole compounds. Sci. Rep. 2021, 11, 2073. [Google Scholar] [CrossRef]
- Ghanbariasad, A.; Emami, L.; Zarenezhad, E.; Behrouz, S.; Zarenezhad, A.; Rad, M.N.S. Synthesis, biological evaluation and in silico studies of 1,2,3−triazolyl−metronidazole derivatives against Leishmania major. New J. Chem. 2022, 46, 8451–8463. [Google Scholar] [CrossRef]
- Britton, R.; Gouverneur, V.; Lin, J. −H.; Meanwell, M.; Ni, C.; Pupo, G.; Xiao, J.−C.; Hu, J. Contemporary synthetic strategies in organofluorine chemistry. Nat. Rev. Methods Primers 2021, 1, 47. [Google Scholar] [CrossRef]
- Mannava, M.K.C.; Bommaka, M.K.; Dandela, R.; Solomon, K.A.; Nangia, A.K. Fluorobenzoic acid coformers to improve the solubility and permeability of the BCS class IV drug naftopidil. Chem. Commun. 2022, 58, 5582–5585. [Google Scholar] [CrossRef]
- Lee, E.; Kamlet, A.S.; Powers, D.C.; Neumann, C.N.; Boursalian, G.B.; Furuya, T.; Choi, D.C.; Hooker, J.M.; Ritter, T. A Fluoride−Derived Electrophilic Late−Stage Fluorination Reagent for PET Imaging. Science 2011, 334, 639–642. [Google Scholar] [CrossRef] [Green Version]
- Bohm, H.J.; Banner, D.; Bendels, S.; Kansy, M.; Kuhn, B.; Muller, K.; Obst−Sander, U.; Stahl, M. Fluorine in medicinal chemistry. ChemBioChem 2004, 5, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Neumann, C.N.; Ritter, T. Introduction of fluorine and fluorine−containing functional groups. Angew. Chem. Int. Ed. Engl. 2013, 52, 8214–8264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashidzume, A.; Nakamura, T.; Sato, T. Copper−catalyzed azide−alkyne cycloaddition oligomerization of 3−azido−1−propyne derivatives. Polymer 2013, 54, 3448–3451. [Google Scholar] [CrossRef]
- Kleinpenning, F.; Steigenberger, B.; Wu, W.; Heck, A.J.R. Fishing for newly synthesized proteins with phosphonate−handles. Nat. Commun. 2020, 11, 3244. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Tolentino, D.R.; Melaimi, M.; Bertrand, G. Isolation of bis(copper) key intermediates in Cu−catalyzed azide−alkyne “Click reaction”. Sci. Adv. 2015, 1, e1500304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rengasamy, R.; Raj, J.P.; Vijayalakshmi, K.; Punitha, N.; Kesavan, M.; Vajjiravel, M.; Elangovan, J. Tunable Synthesis of 1,2,3-Triazoles and Enamines through Deacylative Azide-Alkene Cycloaddition. Eur. J. Org. Chem. 2022, 2022, e202101470. [Google Scholar] [CrossRef]
- Carvalho, L.L.; Bittencourt Pena, R.; Correia Romeiro, N.; Nepomuceno-Silva, J.L. A Concise Synthesis of Triazole Analogues of Lavendustin A via Click Chemistry Approach and Preliminary Evaluation of Their Antiparasitic Activity against Trypanosoma cruzi. ChemistrySelect 2022, 7, e202200128. [Google Scholar] [CrossRef]
- Zhang, C.; Zheng, L.; Yan, Q.; Hu, Q.; Jia, F.; Chen, Y. A Direct P2O5−Mediated Synthesis of Diverse Sulfur−Containing Triazoles via Alkylation of NH−1,2,3−triazoles with Dimethyl Sulfoxide. ChemistrySelect 2018, 3, 10277–10280. [Google Scholar] [CrossRef]
- Damavandi, F.; Wang, W.; Shen, W.Z.; Cetinel, S.; Jordan, T.; Jovel, J.; Montemagno, C.; Wong, G.K. Enrichment of low abundance DNA/RNA by oligonucleotide−clicked iron oxide nanoparticles. Sci. Rep. 2021, 11, 13053. [Google Scholar] [CrossRef]
- Dhyani, A.; Wang, J.; Halvey, A.K.; Macdonald, B.; Mehta, G.; Tuteja, A. Design and applications of surfaces that control the accretion of matter. Science 2021, 373, aba5010. [Google Scholar] [CrossRef]
- Larsson, A.I.; Mattsson−Thorngren, L.; Granhag, L.M.; Berglin, M. Fouling−release of barnacles from a boat hull with comparison to laboratory data of attachment strength. J. Exp. Mar. Biol. Ecol. 2010, 392, 107–114. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Wang, G.; Lei, L.; Xiong, Y.; Fang, Z.; Huang, L.; Liu, J.; Hu, D.; Liao, J. Fluorinated-Triazole-Modified ZnO and Its Application in Marine Antifouling. Coatings 2022, 12, 855. https://doi.org/10.3390/coatings12060855
Yang Y, Wang G, Lei L, Xiong Y, Fang Z, Huang L, Liu J, Hu D, Liao J. Fluorinated-Triazole-Modified ZnO and Its Application in Marine Antifouling. Coatings. 2022; 12(6):855. https://doi.org/10.3390/coatings12060855
Chicago/Turabian StyleYang, Yu, Guoqing Wang, Longlin Lei, Yangkai Xiong, Zhiqiang Fang, Lei Huang, Jinbo Liu, Daxiong Hu, and Jianhe Liao. 2022. "Fluorinated-Triazole-Modified ZnO and Its Application in Marine Antifouling" Coatings 12, no. 6: 855. https://doi.org/10.3390/coatings12060855
APA StyleYang, Y., Wang, G., Lei, L., Xiong, Y., Fang, Z., Huang, L., Liu, J., Hu, D., & Liao, J. (2022). Fluorinated-Triazole-Modified ZnO and Its Application in Marine Antifouling. Coatings, 12(6), 855. https://doi.org/10.3390/coatings12060855