Atmospheric Plasma and UV Polymerisation for Developing Sustainable Anti-Adhesive Polyethylene Terephthalate (PET) Surfaces
Abstract
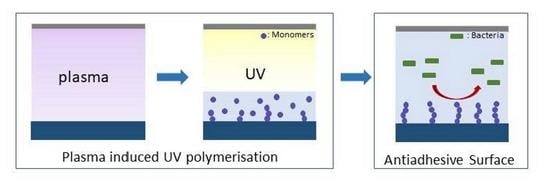
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pre-Treatment with Atmospheric Plasma
2.3. Experimental Design and Optimisation by Response Surface Methodology (RSM)
2.4. UV Polymerisation of PET with Acrylates
2.5. Water Contact Angle (WCA)
2.6. XPS Analysis
2.7. UV-VIS Spectrophotometer
2.8. AFM Analysis
2.9. Anti-Adhesive Performance
3. Results and Discussion
3.1. Pre-Treatment of the PET Substrate
3.2. Grafting of PET with Acrylates
3.3. PET Anti-Adhesiveness
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Suetens, C.; Latour, K.; Kärki, T.; Ricchizzi, E.; Kinross, P.; Moro, M.L.; Jans, B.; Hopkins, S.; Hansen, S.; Lyytikäinen, O.; et al. Prevalence of healthcare-associated infections, estimated incidence and composite antimicrobial resistance index in acute care hospitals and long-term care facilities: Results from two european point prevalence surveys, 2016 to 2017. Eurosurveillance 2018, 23, 1800516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Çaykara, T.; Sande, M.G.; Azoia, N.; Rodrigues, L.R.; Silva, C.J. Exploring the potential of polyethylene terephthalate in the design of antibacterial surfaces. Med. Microbiol. Immunol. 2020, 209, 363–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damodaran, V.B.; Murthy, N.S. Bio-inspired strategies for designing antifouling biomaterials. Biomater. Res. 2016, 20, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domonkos, M.; Tichá, P.; Trejbal, J.; Demo, P. Applications of Cold Atmospheric Pressure Plasma Technology in Medicine, Agriculture and Food Industry. Appl. Sci. 2021, 11, 4809. [Google Scholar] [CrossRef]
- Junkar, I.; Modic, M.; Mozeti, M. Modification of PET surface properties using extremely non-equilibrium oxygen plasma. Open Chem. 2015, 13, 490–496. [Google Scholar] [CrossRef]
- Rezaei, F.; Dickey, M.D.; Bourham, M.; Hauser, P.J. Surface modification of PET film via a large area atmospheric pressure plasma: An optical analysis of the plasma and surface characterization of the polymer film. Surf. Coat. Technol. 2017, 309, 371–381. [Google Scholar] [CrossRef]
- Katsikogianni, M.; Amanatides, E.; Mataras, D.; Missirlis, Y.F. Staphylococcus epidermidis adhesion to He, He/O2 plasma treated PET films and aged materials: Contributions of surface free energy and shear rate. Coll. Surf. B Biointerfaces 2008, 65, 257–268. [Google Scholar] [CrossRef]
- Vazirinasab, E.; Jafari, R.; Momen, G. Evaluation of atmospheric-pressure plasma parameters to achieve superhydrophobic and self-cleaning HTV silicone rubber surfaces via a single-step, eco-friendly approach. Surf. Coat. Technol. 2019, 375, 100–111. [Google Scholar] [CrossRef]
- Scherzer, T. VUV-Induced Photopolymerization of Acrylates. Macromol. Chem. Phys. 2012, 213, 324–334. [Google Scholar] [CrossRef]
- Bitar, R.; Cools, P.; De Geyter, N.; Morent, R. Acrylic acid plasma polymerization for biomedical use. Appl. Surf. Sci. 2018, 448, 168–185. [Google Scholar] [CrossRef]
- Nikiforov, A.; Ma, C.; Choukourov, A.; Palumbo, F. Plasma Technology in Antimicrobial Surface Engineering. J. Appl. Phys. 2022, 131, 011102. [Google Scholar] [CrossRef]
- Arens, L.; Barther, D.; Landsgesell, J.; Holm, C.; Wilhelm, M. Poly(sodium acrylate) hydrogels: Synthesis of various network architectures, local molecular dynamics, salt partitioning, desalination and simulation. Soft Matter 2019, 15, 9949–9964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharyya, R.; Chowdhury, P. Hydrogels of Acryloyl guar gum-g-(acrylic acid-co-3sulfopropylacrylate) for high-performance adsorption and release of gentamicin sulphate. J. Polym. Res. 2021, 28, 286. [Google Scholar] [CrossRef]
- Ghasri, M.; Bouhendi, H.; Kabiri, K.; Zohuriaan-Mehr, M.J.; Karami, Z.; Omidian, H. Superabsorbent polymers achieved by surface cross linking of poly (sodium acrylate) using microwave method. Iran. Polym. J. 2019, 28, 539–548. [Google Scholar] [CrossRef]
- Kalin, M.; Polajnar, M. The wetting of steel, DLC coatings, ceramics and polymers with oils and water: The importance and correlations of surface energy, surface tension, contact angle and spreading. Appl. Surf. Sci. 2014, 293, 97–108. [Google Scholar] [CrossRef]
- Swanepoel, R. Determination of the thickness and optical constants of amorphous silicon. J. Phys. E Sci. Instrum. 1983, 16, 1214. [Google Scholar] [CrossRef] [Green Version]
- Caykara, T.; Silva, J.; Fernandes, S.; Braga, A.; Rodrigues, J.; Rodrigues, L.R.; Silva, C. Modification of PET surfaces with gum Arabic towards its bacterial anti- adhesiveness using an experimental factorial design approach. Mater. Today Commun. 2021, 28, 102684. [Google Scholar] [CrossRef]
- Rodrigues, J.L.; Sousa, M.; Prather, K.L.J.; Kluskens, L.D.; Rodrigues, L.R. Selection of Escherichia coli heat shock promoters toward their application as stress probes. J. Biotechnol. 2014, 188, 61–71. [Google Scholar] [CrossRef] [Green Version]
- Wollmann, P.; Zeth, K.; Lupas, A.N.; Linke, D. Purification of the YadA membrane anchor for secondary structure analysis and crystallization. Int. J. Biol. Macromol. 2006, 39, 3–9. [Google Scholar] [CrossRef]
- Morent, R.; De Geyter, N.; Leys, C.; Gengembre, L.; Payen, E. Study of the ageing behaviour of polymer films treated with a dielectric barrier discharge in air, helium and argon at medium pressure. Surf. Coat. Technol. 2007, 201, 7847–7854. [Google Scholar] [CrossRef]
- Vesel, A.; Mozetic, M. Surface modi fi cation and ageing of PMMA polymer by oxygen plasma treatment. Vaccum 2012, 86, 634–637. [Google Scholar] [CrossRef]
- Contini, C.; Katsikogianni, M.G.; O’Neill, F.T.; O’Sullivan, M.; Boland, F.; Dowling, D.P.; Monahan, F.J. Storage Stability of an Antioxidant Active Packaging Coated with Citrus Extract Following a Plasma Jet Pretreatment Storage Stability of an Antioxidant Active Packaging Coated with Citrus Extract Following a Plasma Jet Pretreatment. Food Bioprocess Technol. 2014, 7, 2228–2240. [Google Scholar] [CrossRef]
- Wang, C.; Chen, J. Studies on surface graft polymerization of acrylic acid onto PTFE film by remote argon plasma initiation. Appl. Surf. Sci. 2007, 253, 4599–4606. [Google Scholar] [CrossRef]
- Backmann, N.; Kappeler, N.; Braun, T.; Huber, F.; Lang, H.P.; Gerber, C.; Lim, R.Y.H. Sensing surface PEGylation with microcantilevers. Beilstein J. Nanotechnol. 2010, 1, 3–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, S.; Zhang, X.; Yu, B.; Zhou, F. Brushing up functional materials. NPG Asia Mater. 2019, 11, 24. [Google Scholar] [CrossRef]
- Zeng, Y.; Xie, L.; Chi, F.; Liu, D.; Wu, H.; Pan, N.; Sun, G. Controlled Growth of Ultra-Thick Polymer Brushes via Surface-Initiated Atom Transfer Radical Polymerization with Active Polymers as Initiators. Macromol. Rapid Commun. 2019, 40, 1900078. [Google Scholar] [CrossRef] [PubMed]
- Berne, C.; Ducret, A.; Hardy, G.G.; Brun, Y.V. Adhesins Involved in Attachment to Abiotic Surfaces by Gram-Negative Bacteria. Microbiol. Spectr. 2015, 3, MB-0018-2015. [Google Scholar] [CrossRef] [Green Version]
- Schmid, Y.; Grassl, G.A.; Bühler, O.T.; Skurnik, M.; Autenrieth, I.B.; Bohn, E. Yersinia enterocolitica adhesin A induces production of interleukin-8 in epithelial cells. Infect. Immun. 2004, 72, 6780–6789. [Google Scholar] [CrossRef] [Green Version]
- Maan, A.M.C.; Hofman, A.H.; De Vos, W.M.; Kamperman, M. Recent Developments and Practical Feasibility of Polymer-Based Antifouling Coatings. Adv. Funct. Mater. 2020, 30, 2000936. [Google Scholar] [CrossRef]








| Independent Variables | Factor | Range and Levels | ||
|---|---|---|---|---|
| −1 | 0 | +1 | ||
| Speed (m/min) | A | 4 | 8 | 12 |
| Power (kW) | B | 7.5 | 11.25 | 15 |
| Sample | Speed (m/min) | Power (kW) | Water Contact Angle (°) | Surface Energy (mJ/m2) | Average Surface Roughness (nm) | Surface Chemistry | |||
|---|---|---|---|---|---|---|---|---|---|
| Initial WCA | SFE Dispersed | SFE Polar | SFE Total | Ra | O/C Ratio | O Increase (%) | |||
| 1 | 4 | 7.5 | 32 ± 1 | 49 ± 1 | 23 ± 1 | 72.5 ± 1 | 5 ± 2 | 0.45 | 40.62 |
| 2 | 4 | 15 | 19 ± 4 | 49 ± 1 | 28 ± 1 | 77 ± 1 | 3.95 ± 0.03 | 0.46 | 43.75 |
| 3 | 12 | 7.5 | 37 ± 10 | 49 ± 1 | 21 ± 5 | 70 ± 5 | 4 ± 1 | 0.37 | 15.62 |
| 4 | 12 | 15 | 25 ± 4 | 49 ± 1 | 26 ± 2 | 75 ± 2 | 4 ± 1 | 0.41 | 28.12 |
| 5 | 8 | 11.25 | 28 ± 4 | 49 ± 1 | 25 ± 2 | 74 ± 2 | 5 ± 2 | 0.37 | 15.62 |
| 6 | 8 | 11.25 | 28 ± 3 | 50 ± 1 | 29 ± 3 | 79 ± 4 | 4 ± 3 | 0.36 | 12.5 |
| 7 | 8 | 11.25 | 30 ± 5 | 49 ± 0.5 | 24 ± 2 | 73 ± 2 | 7 ± 1 | 0.4 | 25 |
| Untreated PET | 0 | 0 | 70 ± 6 | 42 ± 3 | 7 ± 3 | 49 ± 4 | 3 ± 1 | 0.32 | 0 |
| Source | Sum of Squares | Degree of Freedom | Mean Square | F-Value | p–Value | Statistical Significance |
|---|---|---|---|---|---|---|
| Model | 186.5 | 2 | 93.25 | 116.04 | 0.0003 | Significant |
| A-speed | 30.25 | 1 | 30.25 | 37.64 | 0.0036 | – |
| B-power | 156.25 | 1 | 156.25 | 194.44 | 0.0002 | – |
| Residual | 3.21 | 4 | 0.8036 | – | – | – |
| Lack of fit | 0.5476 | 2 | 0.2738 | 0.2054 | 0.8296 | Not significant |
| Pure error | 2.67 | 2 | 1.33 | – | – | – |
| Cor total | 189.71 | 6 | – | – | – | – |
| C 1s | O 1s | O/C Ratio | |||||
|---|---|---|---|---|---|---|---|
| Peak Deconvolution | C1 | C2 | C3 | C4 | O1 | O2 | – |
| Functional groups | C-C and C=C | -C-O- | O-C=O | -C=C | O=C | O-C | – |
| Binding energy (eV) | 285.00 | 286.58 | 288.99 | 291.53 | 531.93 | 533.53 | – |
| Untreated PET | 44.96 | 15.18 | 13.12 | 2.28 | 11.08 | 13.03 | 0.32 |
| Plasma-treated PET with 7.5 kW power and 4 m/min speed | 37.33 | 14.25 | 14.16 | 3.17 | 12.74 | 17.99 | 0.45 |
| Plasma-treated PET with 7.5 kW power and 12 m/min speed | 39.48 | 15.94 | 14.18 | 2.34 | 10.69 | 16.12 | 0.37 |
| Plasma-treated PET with 15 kW power and 4 m/min speed | 35.29 | 15.94 | 14.3 | 2.27 | 15.99 | 15.52 | 0.46 |
| Plasma-treated PET with 15 kW power and 12 m/min speed | 38 | 16.56 | 14.06 | 1.95 | 12.43 | 16.49 | 0.41 |
| Plasma-treated PET with 11.25 kW power and 8 m/min speed | 43.46 | 15.09 | 12.78 | 1.59 | 12.93 | 14.3 | 0.38 |
| Samples | Initial WCA (°) | WCA after 5 Washing Cycles (°) | WCA after 10 Washing Cycles (°) | WCA after 1 Week (°) | WCA after 2 Weeks (°) | WCA after 3 Weeks (°) | SFE Dispersed (mJ/m2) | SFE Polar (mJ/m2) | SFE Total (mJ/m2) | Ra (nm) |
|---|---|---|---|---|---|---|---|---|---|---|
| Untreated PET | 70 ± 6 | N/A | N/A | N/A | N/A | N/A | 42 ± 3 | 7 ± 3 | 49 ± 4 | 2.7 ± 0.9 |
| NaAc-grafted PET | 8 ± 3 | 8 ± 3 | 14 ± 9 | 14 ± 4 | 17 ± 9 | 16 ± 8 | 37 ± 3 | 38 ± 2 | 75 ± 1 | 3 ± 1 |
| Kac-grafted PET | 28 ± 13 | 33 ± 10 | 55 ± 27 | 30 ± 11 | 30 ± 8 | 28 ± 10 | 33 ± 3 | 33 ± 7 | 66 ± 7 | 2.4 ± 0.3 |
| No-plasma NaAc-grafted PET | 39 ± 25 | 54 ± 12 | 58 ± 10 | – | – | – | – | – | – | – |
| No-plasma Kac-grafted PET | 23 ± 18 | 58 ± 16 | 77 ± 7 | – | – | – | – | – | – | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caykara, T.; Fernandes, S.; Braga, A.; Rodrigues, J.; Rodrigues, L.R.; Silva, C.J. Atmospheric Plasma and UV Polymerisation for Developing Sustainable Anti-Adhesive Polyethylene Terephthalate (PET) Surfaces. Coatings 2023, 13, 715. https://doi.org/10.3390/coatings13040715
Caykara T, Fernandes S, Braga A, Rodrigues J, Rodrigues LR, Silva CJ. Atmospheric Plasma and UV Polymerisation for Developing Sustainable Anti-Adhesive Polyethylene Terephthalate (PET) Surfaces. Coatings. 2023; 13(4):715. https://doi.org/10.3390/coatings13040715
Chicago/Turabian StyleCaykara, Tugce, Sara Fernandes, Adelaide Braga, Joana Rodrigues, Ligia Raquel Rodrigues, and Carla Joana Silva. 2023. "Atmospheric Plasma and UV Polymerisation for Developing Sustainable Anti-Adhesive Polyethylene Terephthalate (PET) Surfaces" Coatings 13, no. 4: 715. https://doi.org/10.3390/coatings13040715
APA StyleCaykara, T., Fernandes, S., Braga, A., Rodrigues, J., Rodrigues, L. R., & Silva, C. J. (2023). Atmospheric Plasma and UV Polymerisation for Developing Sustainable Anti-Adhesive Polyethylene Terephthalate (PET) Surfaces. Coatings, 13(4), 715. https://doi.org/10.3390/coatings13040715