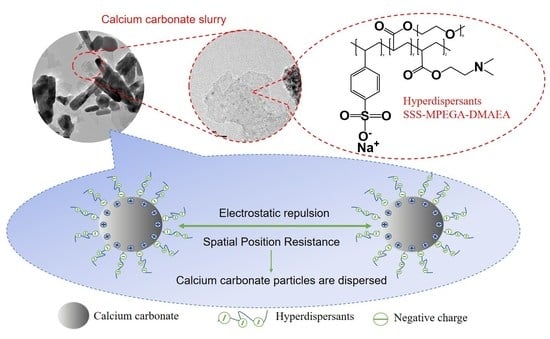
Synthesis of CaCO3-Based Hyperdispersants and Their Application in Aqueous Coatings
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of Copolymer SSS–MPEGA–DMAEA
2.3. Formulation of Pigment Pastes and Coatings
2.4. Central Composite Design of Hyperdispersant Monomer Ratios
2.5. Testing and Characterization
2.5.1. Characterization of the Hyperdispersant SSS–MPEGA–DMAEA
2.5.2. Hyperdispersant Dispersion Performance Test
2.5.3. Paint Suspension Rate Stability Test
2.5.4. Coating Viscosity Change Test
2.5.5. Water Resistance Test of Paint Film
2.5.6. Acid Resistance Test of Paint Film
2.5.7. Alkali Resistance Test of Paint Film
2.5.8. Environmental Scanning Electron Microscope Test of Paint Film
3. Results and Discussion
3.1. Central Composite Design
3.2. Characterization of Hyperdispersant SSS–MPEGA–DMAEA
3.2.1. Nuclear Magnetic Resonance Hydrogen Spectroscopy (1H NMR) Analysis
3.2.2. Gel Permeation Chromatography (GPC) Analysis
3.2.3. Particle Size Analysis
3.3. Optimal Amount of Hyperdispersant
3.4. Coating Performance Characterization
3.4.1. Stability of Coating Suspension Rate
3.4.2. Coating Viscosity Stability
3.4.3. Water Resistance of Paint Film
3.4.4. Acid Resistance of Paint Film
3.4.5. Alkali Resistance of Paint Film
3.4.6. SEM Analysis of Paint Film Surface
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hong, T.; He, Y.; Xu, Z. Preparation and characterization of calcium carbonate–titanium dioxide core–shell (CaCO3@TiO2) nanoparticles and application in the papermaking industry. Powder Technol. 2015, 283, 308–314. [Google Scholar] [CrossRef]
- Alali, F.M.; Tarakji, B.; Alqahtani, A.S.; Alqhtani, N.R.; Nabhan, A.B.; Alenzi, A.; Alrafedah, A.; Robaian, A.; Noushad, M.; Kujan, O.; et al. Characterization of superhydrophobic epoxy coatings embedded by modified calcium carbonate nanoparticles. Prog. Org. Coat. 2016, 101, 577–586. [Google Scholar] [CrossRef]
- Ghari, H.S.; Jalali-Arani, A. Nanocomposites based on natural rubber, organoclay and nano-calcium carbonate: Study on the structure, cure behavior, static and dynamic-mechanical properties. Appl. Clay Sci. 2016, 119, 348–357. [Google Scholar] [CrossRef]
- Rudawska, A.; Frigione, M. Cold-Cured Bisphenolic Epoxy Adhesive Filled with Low Amounts of CaCO3: Effect of the Filler on the Durability to Aqueous Environments. Materials 2021, 14, 1324. [Google Scholar] [CrossRef]
- Li, Y.; Gu, W.J.; He, B.G. Investigation into Coating Surface Free Energy and Microstructure of Coated Paper Related to Pigment. In Advanced Materials Research; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2014; Volume 884, pp. 212–215. [Google Scholar] [CrossRef]
- Wang, J.; Feng, Y.; Xie, J.; Li, G.; Li, X. Dispersion of phthalocyanine green G in nonaqueous medium using hyperdispersants and application in E-ink. J. Dispers. Sci. Technol. 2006, 27, 975–981. [Google Scholar] [CrossRef]
- Farrokhpay, S. A review of polymeric dispersant stabilisation of titania pigment. Adv. Colloid Interface Sci. 2009, 151, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Sis, H.; Birinci, M. Wetting and rheological characteristics of hydrophobic organic pigments in water in the presence of non-ionic surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2014, 455, 58–66. [Google Scholar] [CrossRef]
- Liu, W.; Cheng, L.; Yao, X.; Fang, R.; Liu, G. Synthesis and properties of novel acrylic polyester hyper-dispersant. J. Coat. Technol. Res. 2016, 13, 763–768. [Google Scholar] [CrossRef]
- Bayer, I.S. Superhydrophobic coatings from ecofriendly materials and processes: A review. Adv. Mater. Interfaces 2020, 7, 2000095. [Google Scholar] [CrossRef]
- Barrino, F.; De La Rosa-Ramírez, H.; Schiraldi, C.; López-Martínez, J.; Samper, M.D. Preparation and Characterization of New Bioplastics Based on Polybutylene Succinate (PBS). Polymers 2023, 15, 1212. [Google Scholar] [CrossRef]
- Qian, T.; Zhong, Y.; Mao, Z.; Xu, H.; Zhang, L.; Sui, X.; Wang, B. The comb-like modified styrene-maleic anhydride copolymer dispersant for disperse dyes. J. Appl. Polym. Sci. 2019, 136, 47330. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, J.; Du, C.; Fu, S.; Liu, X. Preparation of nanoscale carbon black dispersion using hyper-branched poly (styrene-alt-maleic anhydride). Prog. Org. Coat. 2012, 75, 537–542. [Google Scholar] [CrossRef]
- Tian, Q.; Zhang, Q. Synthesis and performance of sodium salt of MAA/St/SAS copolymer dispersant. IOP Conf. Ser. Earth Environ. Sci. IOP Publ. 2019, 310, 042032. [Google Scholar] [CrossRef]
- Zhang, G.; Zhu, N.; Zhu, X. Influence of Polycarboxylate Dispersants with Different Molecular Structures on the Performance of Coal Water Slurry. J. Dispers. Sci. Technol. 2016, 37, 1799–1805. [Google Scholar] [CrossRef]
- Du, L.; Zhang, G.; Yang, D.; Luo, J.; Liu, Y.; Zhang, W.; Zhang, C.; Li, J.; Zhu, J. Synthesis of a novel amphoteric copolymer and its application as a dispersant for coal water slurry preparation. R. Soc. Open Sci. 2021, 8, 201480. [Google Scholar] [CrossRef]
- Lu, X.; Xia, Y.; Liu, M.; Qian, Y.; Zhou, X.; Gu, N. Improved performance of diatomite-based dental nanocomposite ceramics using layer-by-layer assembly. Int. J. Nanomed. 2012, 7, 2153. [Google Scholar] [CrossRef]
- Obata, M.; Tanaka, S.; Mizukoshi, H.; Ishihara, E.; Takahashi, M.; Hirohara, S. RAFT synthesis of polystyrene-block-poly (polyethylene glycol monomethyl ether acrylate) for zinc phthalocyanine-loaded polymeric micelles as photodynamic therapy photosensitizers. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 560–570. [Google Scholar] [CrossRef]
- Touaiti, F.; Alam, P.; Nilsson, R.; Pahlevan, M.; Ansell, M.; Wilen, C.; Toivakka, M. Thermomechanical properties of CaCO3-latex pigment coatings: Impact of modified dispersing agents. Prog. Org. Coat. 2013, 76, 439–446. [Google Scholar] [CrossRef]
- Büyükyağcı, A.; Tuzcu, G.; Aras, L. Synthesis of copolymers of methoxy polyethylene glycol acrylate and 2-acrylamido-2-methyl-1-propanesulfonic acid: Its characterization and application as superplasticizer in concrete. Cem. Concr. Res. 2009, 39, 629–635. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Wang, C.Y.; Ren, Q. RAFT surfactant-free cationic emulsion polymerization of styrene: Effect of hydrophobicity of block macro-RAFT agent. J. Macromol. Sci. Part A 2020, 58, 232–242. [Google Scholar] [CrossRef]
- Liu, S.H.; Wu, T.; Lu, Y.M.; Li, H.-S. Particular thermal behavior of 2, 2′-azobis [2-(2-imidazolin-2-yl) propane] dihydrochloride using DSC and STA. J. Therm. Anal. Calorim. 2021, 144, 343–349. [Google Scholar] [CrossRef]
- Ferreira SL, C.; Lemos, V.A.; de Carvalho, V.S.; da Silva, E.G.; Queiroz, A.F.; Felix, C.S.; da Silva, D.L.; Dourado, G.B.; Oliveira, R.V. Multivariate optimization techniques in analytical chemistry-an overview. Microchem. J. 2018, 140, 176–182. [Google Scholar] [CrossRef]
- Song, J.O.; McCormick, A.V.; Francis, L.F. Depthwise Viscosity Gradients in UV-Cured Epoxy Coatings. Macromol. Mater. Eng. 2013, 298, 145–152. [Google Scholar] [CrossRef]
- Khan, M.; Hamid, A.; Tiehu, L.; Zada, A.; Attique, F.; Ahmad, N.; Ullah, A.; Hayat, A.; Mahmood, I.; Hussain, A.; et al. Surface optimization of detonation nanodiamonds for the enhanced mechanical properties of polymer/nanodiamond composites. Diam. Relat. Mater. 2020, 107, 107897. [Google Scholar] [CrossRef]
- Chakraborty, S.; Sarkar, I.; Behera, D.K.; Pal, S.K.; Chakraborty, S. Experimental investigation on the effect of dispersant addition on thermal and rheological characteristics of TiO2 nanofluid. Powder Technol. 2017, 307, 10–24. [Google Scholar] [CrossRef]
- Khan, M.; Tiehu, L.; Hussain, A.; Raza, A.; Zada, A.; Alei, D.; Khan, A.R.; Ali, R.; Hussain, H.; Hussain, J.; et al. Physiochemical evaluations, mechanical attenuations and thermal stability of graphene nanosheets and functionalized nanodiamonds loaded pitch derived carbon foam composites. Diam. Relat. Mater. 2022, 126, 109077. [Google Scholar] [CrossRef]
- Sethi, S.K.; Manik, G. A combined theoretical and experimental investigation on the wettability of MWCNT filled PVAc-g-PDMS easy-clean coating. Prog. Org. Coat. 2021, 151, 106092. [Google Scholar] [CrossRef]



















| Coating Composition | Acrylic Resin | Dispersants | Defoamer | CaCO3 | TiO2 | PGMEA | Deionized Water |
|---|---|---|---|---|---|---|---|
| Percentage of components (%) | 65 | 0.5 | 0.03 | 15.5 | 8 | 4 | 6.97 |
| Dosage (g) | 32.5 | 0.25 | 0.015 | 7.75 | 4 | 2 | 3.485 |
| Level | Factors | ||
|---|---|---|---|
| Amount of SSS (X1, mol) | Amount of MPEGA (X2, mol) | Amount of DMAEA (X3, mol) | |
| −1.68 | 0.32 | 0.13 | 0.66 |
| −1 | 1 | 0.2 | 1 |
| 0 | 2 | 0.3 | 1.5 |
| 1 | 3 | 0.4 | 2 |
| 1.68 | 3.68 | 0.47 | 2.34 |
| Serial Number | X1 (mol) | X2 (mol) | X3 (mol) | Y (mPa·s) |
|---|---|---|---|---|
| 1 | 3.68 | 0.30 | 1.50 | 102 |
| 2 | 2.00 | 0.30 | 1.50 | 116 |
| 3 | 2.00 | 0.30 | 1.50 | 104 |
| 4 | 3.00 | 0.40 | 1.00 | 115 |
| 5 | 2.00 | 0.30 | 0.66 | 101 |
| 6 | 1.00 | 0.20 | 2.00 | 424 |
| 7 | 3.00 | 0.40 | 2.00 | 139 |
| 8 | 1.00 | 0.20 | 1.00 | 153 |
| 9 | 2.00 | 0.30 | 1.50 | 104 |
| 10 | 1.00 | 0.40 | 2.00 | 529 |
| 11 | 3.00 | 0.20 | 1.00 | 75 |
| 12 | 3.00 | 0.20 | 2.00 | 77 |
| 13 | 2.00 | 0.30 | 1.50 | 105 |
| 14 | 2.00 | 0.47 | 1.50 | 169 |
| 15 | 2.00 | 0.30 | 2.34 | 130 |
| 16 | 2.00 | 0.13 | 1.50 | 104 |
| 17 | 0.32 | 0.30 | 1.50 | 805 |
| 18 | 2.00 | 0.30 | 1.50 | 109 |
| 19 | 1.00 | 0.40 | 1.00 | 362 |
| Parameters | Coefficients and p-Values of the Multiple Linear Regression Equation | ||
|---|---|---|---|
| Independent variables | X1 −89.16 (<0.0001) | X2 45.38 (0.0434) | X3 16.27 (0.0483) |
| Interaction items | X1X2 −65.70 (0.1354) | X1X3 −23.45 (0.0172) | X2X3 1.70 (0.4369) |
| Secondary items | X12 59.70 (<0.0001) | X22 28.16 (0.8418) | X32 12.25 (0.7824) |
| Category | Slurry Viscosity (mPa·s) | |||||
|---|---|---|---|---|---|---|
| Experimental Value | Average Value | Predicted Value | Deviation | |||
| CaCO3 | 55 | 67 | 62 | 61.3 | 60 | 1.3 |
| Test Items | Test Results |
|---|---|
| Sparkling | No visible changes in the two experimental groups within seven days |
| Rusting | No visible changes in the two experimental groups within seven days |
| Flaking | No visible changes in the two experimental groups within seven days |
| Chalking | Both experimental groups showed a small amount of pigment particles precipitated on the sixth day |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, J.; Li, Y. Synthesis of CaCO3-Based Hyperdispersants and Their Application in Aqueous Coatings. Coatings 2023, 13, 819. https://doi.org/10.3390/coatings13050819
Bai J, Li Y. Synthesis of CaCO3-Based Hyperdispersants and Their Application in Aqueous Coatings. Coatings. 2023; 13(5):819. https://doi.org/10.3390/coatings13050819
Chicago/Turabian StyleBai, Jue, and Yu Li. 2023. "Synthesis of CaCO3-Based Hyperdispersants and Their Application in Aqueous Coatings" Coatings 13, no. 5: 819. https://doi.org/10.3390/coatings13050819
APA StyleBai, J., & Li, Y. (2023). Synthesis of CaCO3-Based Hyperdispersants and Their Application in Aqueous Coatings. Coatings, 13(5), 819. https://doi.org/10.3390/coatings13050819



.jpg)