Thermal Sprayed Protective Coatings for Bipolar Plates of Hydrogen Fuel Cells and Water Electrolysis Cells
Abstract
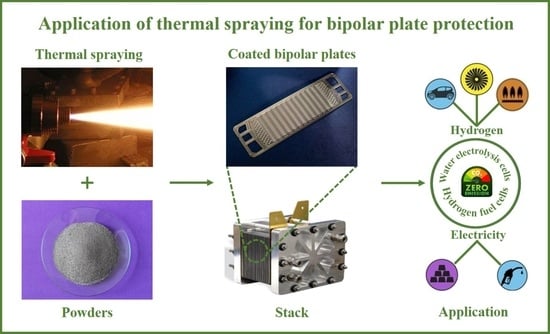
:1. Introduction
2. Atmospheric Plasma Spraying
2.1. Features and Strengths of Atmospheric Plasma Spraying
2.2. Application of APS-Sprayed Coatings in SOFCs

2.2.1. Application of APS-Sprayed Rare Earth Perovskite Oxide Coatings
2.2.2. Application of APS-Sprayed Spinel Oxide Coatings
2.3. Application of APS-Sprayed Coatings in SOECs
3. Vacuum Plasma Spraying
3.1. Features and Strengths of Vacuum Plasma Spraying
3.2. Application of VPS-Sprayed Coatings in PEM Cells
3.2.1. Application of VPS-Sprayed Ti Coatings

3.2.2. Application of VPS-Sprayed Nb Coatings
4. High-Velocity Oxygen Fuel Spraying
4.1. Features and Strengths of High-Velocity Oxygen Fuel Spraying
4.2. Application of HVOF-Sprayed Coatings in PEM Cells
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, K.F.; Jiang, S.P. Review-materials degradation of solid oxide electrolysis cells. J. Electrochem. Soc. 2016, 163, F3070–F3083. [Google Scholar] [CrossRef]
- Teuku, H.; Alshami, I.; Goh, J.; Masdar, M.S.; Loh, K.S. Review on bipolar plates for low-temperature polymer electrolyte membrane water electrolyzer. Int. J. Energy Res. 2021, 45, 20583–20600. [Google Scholar] [CrossRef]
- Dihrab, S.S.; Sopian, K.; Alghoul, M.A.; Sulaiman, M.Y. Review of the membrane and bipolar plates materials for conventional and unitized regenerative fuel cells. Renew. Sust. Energy Rev. 2009, 13, 1663–1668. [Google Scholar] [CrossRef]
- Hermann, A.; Chaudhuri, T.; Spagnol, P. Bipolar plates for PEM fuel cells: A review. Int. J. Hydrogen Energy 2005, 30, 1297–1302. [Google Scholar] [CrossRef]
- Weber, A.; Ivers-Tiffée, E. Materials and concepts for solid oxide fuel cells (SOFCs) in stationary and mobile applications. J. Power Sources 2004, 127, 273–283. [Google Scholar] [CrossRef]
- Antunes, R.A.; Oliveira, M.C.L.; Ett, G.; Ett, V. Corrosion of metal bipolar plates for PEM fuel cells: A review. Int. J. Hydrogen Energy 2010, 35, 3632–3647. [Google Scholar] [CrossRef]
- Yue, M.; Lambert, H.; Pahon, E.; Roche, R.; Jemei, S.; Hissel, D. Hydrogen energy systems: A critical review of technologies, applications, trends and challenges. Renew. Sust. Energy Rev. 2021, 146, 111180. [Google Scholar] [CrossRef]
- Ebbesen, S.D.; Jensen, S.H.; Hauch, A.; Mogensen, M.B. High temperature electrolysis in alkaline cells, solid proton conducting cells, and solid oxide cells. Chem. Rev. 2014, 114, 10697–10734. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.; Tawfik, H.; Mahajan, D. Durability and characterization studies of chromium carbide coated aluminum fuel cell stack. Int. J. Hydrogen Energy 2016, 41, 12273–12284. [Google Scholar] [CrossRef]
- Carmo, M.; Fritz, D.L.; Mergel, J.; Stolten, D. A comprehensive review on PEM water electrolysis. Int. J. Hydrogen Energy 2013, 38, 4901–4934. [Google Scholar] [CrossRef]
- Wang, Y.; Pang, Y.; Xu, H.; Martinez, A.; Chen, K.S. PEM Fuel cell and electrolysis cell technologies and hydrogen infrastructure development—A review. Energy Environ. Sci. 2022, 15, 2288–2328. [Google Scholar] [CrossRef]
- Golkhatmi, S.Z.; Asghar, M.I.; Lund, P.D. A review on solid oxide fuel cell durability: Latest progress, mechanisms, and study tools. Renew. Sust. Energy Rev. 2022, 161, 112339. [Google Scholar] [CrossRef]
- Ardigo, M.R.; Popa, I.; Chevalier, S.; Girardon, P.; Perry, F.; Laucournet, R.; Brevet, A.; Desgranges, C. Effect of coatings on long term behaviour of a commercial stainless steel for solid oxide electrolyser cell interconnect application in H2/H2O atmosphere. Int. J. Hydrogen Energy 2014, 39, 21673–21677. [Google Scholar] [CrossRef]
- Mao, J.W.; Wang, E.H.; Wang, H.W.; Ouyang, M.G.; Chen, Y.P.; Hu, H.R.; Lu, L.G.; Ren, D.S.; Liu, Y.D. Progress in metal corrosion mechanism and protective coating technology for interconnect and metal support of solid oxide cells. Renew. Sust. Energy Rev. 2023, 185, 113597. [Google Scholar] [CrossRef]
- Singh, M.; Zappa, D.; Comini, E. Solid oxide fuel cell: Decade of progress, future perspectives and challenges. Int. J. Hydrogen Energy 2021, 46, 27643–27674. [Google Scholar] [CrossRef]
- Sharaf, O.Z.; Orhan, M.F. An overview of fuel cell technology: Fundamentals and applications. Renew. Sust. Energy Rev. 2014, 32, 810–853. [Google Scholar] [CrossRef]
- Feng, Q.; Yuan, X.Z.; Liu, G.Y.; Wei, B.; Zhang, Z.; Li, H.; Wang, H.J. A review of proton exchange membrane water electrolysis on degradation mechanisms and mitigation strategies. J. Power Sources 2017, 366, 33–55. [Google Scholar] [CrossRef]
- Lee, H.; Kim, U.S.; Kim, S.D.; Woo, S.K.; Chung, W.J. SiO2-B2O3-BaO-WO3 glasses with varying Al2O3 content as a sealing material for reversible solid oxide fuel cells. Ceram. Int. 2020, 46, 18256–18261. [Google Scholar] [CrossRef]
- Liu, D.Y.; Geng, S.J.; Chen, G.; Wang, F.H. NiO/NiFe2O4 dual-layer coating on pre-oxidized SUS 430 steel interconnect. Int. J. Hydrogen Energy 2022, 47, 21462–21471. [Google Scholar] [CrossRef]
- Gago, A.S.; Lettenmeier, P.; Stiber, S.; Ansar, A.S.; Wang, L.; Friedrich, K.A. Cost-effective PEM electrolysis: The quest to achieve superior efficiencies with reduced investment. ECS Trans. 2018, 85, 3–13. [Google Scholar] [CrossRef]
- Otomo, J.; Oishi, J.; Mitsumori, T.; Iwasaki, H.; Yamada, K. Evaluation of cost reduction potential for 1 kW class SOFC stack production: Implications for SOFC technology scenario. Int. J. Hydrogen Energy 2013, 38, 14337–14347. [Google Scholar] [CrossRef]
- Jung, H.Y.; Huang, S.Y.; Ganesan, P.; Popov, B.N. Performance of gold-coated titanium bipolar plates in unitized regenerative fuel cell operation. J. Power Sources 2009, 194, 972–975. [Google Scholar] [CrossRef]
- Carlson, E.J.; Yang, Y.; Fulton, C. Solid Oxide Fuel Cell Manufacturing Cost Model: Simulating Relationships between Performance, Manufac-Turing and Cost of Production; Technical Report; OSTI: Oak Ridge, TN, USA, 2004. [CrossRef]
- Shen, Z.J.; Rong, J.; Yu, X.H. MnxCo3-xO4 spinel coatings: Controlled synthesis and high temperature oxidation resistance behavior. Ceram. Int. 2020, 46, 5821–5827. [Google Scholar] [CrossRef]
- Stiber, S.; Sata, N.; Morawietz, T.; Ansar, S.A.; Jahnke, T.; Lee, J.K.; Bazylak, A.; Fallisch, A.; Gago, A.S.; Friedrich, K.A. A high-performance, durable and low-cost proton exchange membrane electrolyser with stainless steel components. Energy Environ. Sci. 2022, 15, 109–122. [Google Scholar] [CrossRef]
- Jiang, S.P.; Chen, X.B. Chromium deposition and poisoning of cathodes of solid oxide fuel cells-A review. Int. J. Hydrogen Energy 2014, 39, 505–531. [Google Scholar] [CrossRef]
- Lædre, S.; Mendizabal, L.; Kongstein, O.E.; Oedegaard, A.; Karoliussen, H.; Seland, F. Ta-ITO Coated titanium bipolar plates for proton exchange membrane water electrolyzers. J. Electrochem. Soc. 2022, 169, 034504. [Google Scholar] [CrossRef]
- Fan, H.Q.; Shi, D.D.; Wang, X.Z.; Luo, J.L.; Zhang, J.Y.; Li, Q. Enhancing through-plane electrical conductivity by introducing Au microdots onto TiN coated metal bipolar plates of PEMFCs. Int. J. Hydrogen Energy 2020, 45, 29442–29448. [Google Scholar] [CrossRef]
- Zhu, J.H.; Chesson, D.A.; Yu, Y.T. Review-(Mn,Co)3O4-based spinels for SOFC interconnect coating application. J. Electrochem. Soc. 2021, 168, 114519. [Google Scholar] [CrossRef]
- Salehmin, M.N.I.; Husaini, T.; Goh, J.; Sulong, A.B. High-pressure PEM water electrolyser: A review on challenges and mitigation strategies towards green and low-cost hydrogen production. Energy Convers. Manag. 2022, 268, 115985. [Google Scholar] [CrossRef]
- Gago, A.S.; Ansar, S.A.; Saruhan, B.; Schulz, U.; Lettenmeier, P.; Canas, N.A.; Gazdzicki, P.; Morawietz, T.; Hiesgen, R.; Arnold, J.; et al. Protective coatings on stainless steel bipolar plates for proton exchange membrane (PEM) electrolysers. J. Power Sources 2016, 307, 815–825. [Google Scholar] [CrossRef]
- Gago, A.S.; Ansar, A.S.; Gazdzicki, P.; Wagner, N.; Arnold, J.; Friedrich, K.A. Low cost bipolar plates for large scale PEM electrolyzers. ECS Trans. 2014, 64, 1039–1048. [Google Scholar] [CrossRef]
- Lettenmeier, P.; Wang, R.; Abouatallah, R.; Saruhan, B.; Freitag, O.; Gazdzicki, P.; Morawietz, T.; Hiesgen, R.; Gago, A.S.; Friedrich, K.A. Low-cost and durable bipolar plates for proton exchange membrane electrolyzers. Sci. Rep. 2017, 7, 44035. [Google Scholar] [CrossRef] [PubMed]
- Gago, A.S.; Ansar, A.S.; Wagner, N.; Arnold, J.; Friedrich, K.A. Titanium Coatings Deposited by Thermal Spraying for Bipolar Plates of PEM Electrolysers 4th European PEFC and H2 Forum. Lucerne Switzerland. 2013. Available online: https://elib.dlr.de/92736/1/Chapter-06_EFCF-2013-Session-A07.pdf (accessed on 13 February 2024).
- Feng, K.; Li, Z.G.; Sun, H.L.; Yu, L.; Cai, X.; Wu, Y.X.; Chu, P.K. C/CrN multilayer coating for polymer electrolyte membrane fuel cell metallic bipolar plates. J. Power Sources 2013, 222, 351–358. [Google Scholar] [CrossRef]
- Zhang, D.M.; Duan, L.T.; Guo, L.; Wang, Z.Y.; Zhao, J.; Tuan, W.H.; Niihara, K. TiN-coated titanium as the bipolar plate for PEMFC by multi-arc ion plating. Int. J. Hydrogen Energy 2011, 36, 9155–9161. [Google Scholar] [CrossRef]
- Yoo, J.; Woo, S.K.; Yu, J.H.; Lee, S.; Park, G.W. La0.8Sr0.2MnO3 and (Mn1.5Co1.5)O4 double layer coated by electrophoretic deposition on Crofer22 APU for SOEC interconnect applications. Int. J. Hydrogen Energy 2009, 34, 1542–1547. [Google Scholar] [CrossRef]
- Hui, R.; Wang, Z.W.; Kesler, O.; Rose, L.; Jankovic, J.; Yick, S.; Maric, R.; Ghosh, D. Thermal plasma spraying for SOFCs: Applications, potential advantages, and challenges. J. Power Sources 2007, 170, 308–323. [Google Scholar] [CrossRef]
- Stiber, S.; Hehemann, M.; Carmo, M.; Müller, M.; Ayers, K.E.; Capuano, C.; Danilovic, N.; Morawietz, T.; Biswas, I.; Gazdzicki, P.; et al. Long-term operation of Nb-coated stainless steel bipolar plates for proton exchange membrane water electrolyzers. Adv. Energy Sustain. Res. 2022, 3, 2200024. [Google Scholar] [CrossRef]
- Piccardo, P.; Spotorno, R.; Geipel, C. Investigation of a metallic interconnect extracted from an SOFC stack after 40,000 h of operation. Energies 2022, 15, 3548. [Google Scholar] [CrossRef]
- Hu, Y.Z.; Yao, S.W.; Li, C.X.; Li, C.J.; Zhang, S.L. Influence of pre-reduction on microstructure homogeneity and electrical properties of APS Mn1.5Co1.5O4 coatings for SOFC interconnects. Int. J. Hydrogen Energy 2017, 42, 27241–27253. [Google Scholar] [CrossRef]
- Butt, M.A. Thin-film coating methods: A successful marriage of high-quality and cost-effectiveness-A brief exploration. Coatings 2022, 12, 1115. [Google Scholar] [CrossRef]
- Oksa, M.; Turunen, E.; Suhonen, T.; Varis, T.; Hannula, S.P. Optimization and characterization of high velocity oxy-fuel sprayed coatings: Techniques, materials, and applications. Coatings 2011, 1, 17–52. [Google Scholar] [CrossRef]
- Tejero-Martin, D.; Rad, M.R.; Mcdonald, A.; Hussain, T. Beyond traditional coatings: A review on thermal-sprayed functional and smart coatings. J. Therm. Spray Technol. 2019, 28, 598–644. [Google Scholar] [CrossRef]
- Faisal, N.H.; Prathuru, A.; Ahmed, R.; Cai, Q.; Horri, B.A.; Rajendran, V.; Hossain, M.; Venkatachalapathy, V.; Katiyar, N.K.; Li, J.; et al. Application of thermal spray coatings in electrolysers for hydrogen production advances, challenges, and opportunities. ChemNanoMat 2022, 8, e202200384. [Google Scholar] [CrossRef]
- Han, S.J.; Chen, Y.K.; Sampath, S. Role of process conditions on the microstructure, stoichiometry and functional performance of atmospheric plasma sprayed La(Sr)MnO3 coatings. J. Power Sources 2014, 259, 245–254. [Google Scholar] [CrossRef]
- Yan, Q.; Gambino, R.J.; Sampath, S.; Lewis, L.H.; Li, L.; Baumberger, E.; Vaidya, A.; Xiong, H. Effects of zinc loss on the magnetic properties of plasma-sprayed MnZn ferrites. Acta Mater. 2004, 52, 3347–3353. [Google Scholar] [CrossRef]
- Sampath, S. Thermal spray applications in electronics and sensors: Past, present, and future. J. Therm. Spray Technol. 2010, 19, 921–949. [Google Scholar] [CrossRef]
- Shaigan, N.; Qu, W.; Ivey, D.G.; Chen, W. A review of recent progress in coatings, surface modifications and alloy developments for solid oxide fuel cell ferritic stainless steel interconnects. J. Power Sources 2010, 195, 1529–1542. [Google Scholar] [CrossRef]
- Molin, S.; Jasinski, P.; Mikkelsen, L.; Zhang, W.; Chen, M.; Hendriksen, P.V. Low temperature processed MnCo2O4 and MnCo1.8Fe0.2O4 as effective protective coatings for solid oxide fuel cell interconnects at 750 °C. J. Power Sources 2016, 336, 408–418. [Google Scholar] [CrossRef]
- Mohamed, S.M.; Sanad, M.M.S.; Mattar, T.; El-Shahat, M.F.; Rossignol, C.; Dessemond, L.; Zaidat, K.; Obbade, S. The structural, thermal and electrochemical properties of MnFe1−x-yCuxNiyCoO4 spinel protective layers in interconnects of solid oxide fuel cells (SOFCs). J. Alloys Compd. 2022, 923, 166351. [Google Scholar] [CrossRef]
- Ardigo-Besnard, M.R.; Popa, I.; Chevalier, S. Effect of spinel and perovskite coatings on the long term oxidation of a ferritic stainless steel in H2/H2O atmosphere. Corros. Sci. 2019, 148, 251–263. [Google Scholar] [CrossRef]
- Bianco, M.; Caliandro, P.; Diethelm, S.; Yang, S.; Dellai, A.; Vanherle, J.; Steinberger-Wilckens, R. In-situ experimental benchmarking of solid oxide fuel cell metal interconnect solutions. J. Power Sources 2020, 461, 228163. [Google Scholar] [CrossRef]
- Somalu, M.R.; Muchtar, A.; Daud, W.R.W.; Brandon, N.P. Screen-printing inks for the fabrication of solid oxide fuel cell films: A review. Renew. Sust. Energy Rev. 2017, 75, 426–439. [Google Scholar] [CrossRef]
- Tikkanen, H.; Suciu, C.; Wærnhus, I.; Hoffmann, A.C. Dip-coating of 8YSZ nanopowder for SOFC applications. Ceram. Int. 2011, 37, 2869–2877. [Google Scholar] [CrossRef]
- Torabi, A.; Etsell, T.H.; Sarkar, P. Dip coating fabrication process for micro-tubular SOFCs. Solid State Ion. 2011, 192, 372–375. [Google Scholar] [CrossRef]
- Jonas, G. Deposition via dip coating technique of dense yttrium stabilized zirconia layers. Int. J. Appl. Ceram. Technol. 2013, 10, 79–86. [Google Scholar]
- Hsueh, T.H.; Tsai, C.H.; Liu, S.E.; Wang, M.C.; Chang, S.M.; Shiue, A.; Chin, K.Y. LiCoO2 battery electrode fabricated by high deposition-rate atmospheric plasma spraying for lithium battery. J. Electrochem. Soc. 2022, 169, 100506. [Google Scholar] [CrossRef]
- Hassan, M.A.; Mamat, O.B.; Mehdi, M. Review: Influence of alloy addition and spinel coatings on Cr-based metallic interconnects of solid oxide fuel cells. Int. J. Hydrogen Energy 2020, 45, 25191–25209. [Google Scholar] [CrossRef]
- Wu, J.W.; Liu, X.B. Recent development of SOFC metallic interconnect. J. Mater. Sci. Technol. 2010, 26, 293–305. [Google Scholar] [CrossRef]
- Mazur, Ł.; Molin, S.; Dąbek, J.; Durczak, K.; Pyzalski, M.; Brylewski, T. Physicochemical properties of Mn1.45Co1.45Cu0.1O4 spinel coating deposited on the Crofer 22 H ferritic steel and exposed to high-temperature oxidation under thermal cycling conditions. J. Therm. Anal. Calorim. 2021, 147, 5649–5666. [Google Scholar] [CrossRef]
- Grünwald, N.; Sohn, Y.J.; Yin, X.n.; Menzler, N.H.; Guillon, O.R.; Vaßen, R. Microstructure and phase evolution of atmospheric plasma sprayed Mn-Co-Fe oxide protection layers for solid oxide fuel cells. J. Eur. Ceram. Soc. 2019, 39, 449–460. [Google Scholar] [CrossRef]
- Rufner, J.; Gannon, P.; White, P.; Deibert, M.; Teintze, S.; Smith, R.; Chen, H. Oxidation behavior of stainless steel 430 and 441 at 800 °C in single (air/air) and dual atmosphere (air/hydrogen) exposures. Int. J. Hydrogen Energy 2008, 33, 1392–1398. [Google Scholar] [CrossRef]
- Yang, Z.; Walker, M.S.; Singh, P.; Stevenson, J.W.; Norby, T. Oxidation behavior of ferritic stainless steels under SOFC interconnect exposure conditions. J. Electrochem. Soc. 2004, 151, B669–B678. [Google Scholar] [CrossRef]
- Park, M.; Shin, J.S.; Lee, S.; Kim, H.J.; An, H.; Ji, H.I.; Kim, H.; Son, J.W.; Lee, J.H.; Kim, B.K.; et al. Thermal degradation mechanism of ferritic alloy (Crofer 22 APU). Corros. Sci. 2018, 134, 17–22. [Google Scholar] [CrossRef]
- Natsuko, S.; Teruhisa, H.; Xiong, Y.P.; Katsuhiko, Y.; Haruo, K.; Brito, M.E.; Yokokawa, H.; Maruyama, T. Structure and transport property of manganese-chromium-iron oxide as a main compound in oxide scales of alloy interconnects for SOFCs. Solid State Ion. 2005, 176, 681–686. [Google Scholar]
- Jiang, S.P.; Zhang, J.P.; Foger, K. Deposition of chromium species at Sr-doped LaMnO3 electrodes in solid oxide fuel cells II. Effect on O2 reduction reaction. J. Electrochem. Soc. 2000, 147, 3195–3205. [Google Scholar] [CrossRef]
- Fontana, S.; Chevalier, S.; Caboche, G. Metallic interconnects for solid oxide fuel cell: Performance of reactive element oxide coating during 10, 20 and 30 months exposure. Oxid. Met. 2012, 78, 307–328. [Google Scholar] [CrossRef]
- Report on the Status of the Solid Oxide Fuel Cell Program. Available online: http://www.energy.gov/sites/prod/files/2019/09/f66/EXEC-2019-002655_Signed%20Report%201.pdf (accessed on 13 February 2024).
- Mazur, Ł.; Ignaczak, J.; Bik, M.; Molin, S.; Sitarz, M.; Aleksander, G.; Brylewski, T. Effectiveness of a dual surface modification of metallic interconnects for application in energy conversion devices. Int. J. Hydrogen Energy 2022, 47, 6295–6311. [Google Scholar] [CrossRef]
- Lim, D.P.; Lim, D.S.; Oh, J.S.; Lyo, I.W. Influence of post-treatments on the contact resistance of plasma-sprayed La0.8Sr0.2MnO3 coating on SOFC metallic interconnector. Surf. Coat. Tech. 2005, 200, 1248–1251. [Google Scholar] [CrossRef]
- Baik, K.H. Effects of plasma-sprayed La0.7Sr0.3MnO3 coating on thermally grown oxide scale and electrical conductivity of Fe-Cr interconnect for SOFCs. J. Electrochem. Soc. 2013, 160, F560–F565. [Google Scholar] [CrossRef]
- Wu, W.; Guan, W.; Wang, G.L.; Liu, W.; Zhang, Q.S.; Chen, T.; Wang, W.G. Evaluation of Ni80Cr20(La0.75Sr0.25)0.95MnO3 dual layer coating on SUS 430 stainless steel used as metallic interconnect for solid oxide fuel cells. Int. J. Hydrogen Energy 2014, 39, 996–1004. [Google Scholar] [CrossRef]
- Puranen, J.; Lagerbom, J.; Hyvarinen, L.; Kylmalahti, M.; Himanen, O.; Pihlatie, M.; Kiviaho, J.; Vuoristo, P. The structure and properties of plasma sprayed iron oxide doped manganese cobalt oxide spinel coatings for SOFC metallic interconnectors. J. Therm. Spray Technol. 2010, 20, 154–159. [Google Scholar] [CrossRef]
- Han, S.J.; Pala, Z.; Sampath, S. Plasma sprayed manganese-cobalt spinel coatings: Process sensitivity on phase, electrical and protective performance. J. Power Sources 2016, 304, 234–243. [Google Scholar] [CrossRef]
- Hu, Y.Z.; Gao, J.T.; Li, C.X.; Li, C.J. Thermally sprayed MCO/FeCr24 interconnector with improved stability for tubular segmented-in-series SOFCs. Appl. Surf. Sci. 2022, 587, 152861. [Google Scholar] [CrossRef]
- Waluyo, N.S.; Park, S.S.; Song, R.H.; Lee, S.B.; Lim, T.H.; Hong, J.E.; Ryu, K.H.; Im, W.B.; Lee, J.W. Protective coating based on manganese-copper oxide for solid oxide fuel cell interconnects: Plasma spray coating and performance evaluation. Ceram. Int. 2018, 44, 11576–11581. [Google Scholar] [CrossRef]
- Tomas, M.; Asokan, V.; Puranen, J.; Svensson, J.-E.; Froitzheim, J. Efficiencies of cobalt- and copper-based coatings applied by different deposition processes for applications in intermediate-temperature solid oxide fuel cells. Int. J. Hydrogen Energy 2022, 47, 32628–32640. [Google Scholar] [CrossRef]
- Yang, Z.G.; Xia, G.G.; Maupin, G.D.; Stevenson, J.W. Conductive protection layers on oxidation resistant alloys for SOFC interconnect applications. Surf. Coat. Tech. 2006, 201, 4476–4483. [Google Scholar] [CrossRef]
- Hu, Y.Z.; Li, C.X.; Yang, G.J.; Li, C.J. Evolution of microstructure during annealing of Mn1.5Co1.5O4 spinel coatings deposited by atmospheric plasma spray. Int. J. Hydrogen Energy 2014, 39, 13844–13851. [Google Scholar] [CrossRef]
- Tsai, S.Y.; Fung, K.Z.; Ni, C.T.; Ho, H.C. Dependence of electrical properties on thermal reduction of protecting oxides for SOFC interconnect applications. ECS Trans. 2015, 68, 1641–1647. [Google Scholar] [CrossRef]
- Grünwald, N.; Sebold, D.; Sohn, Y.J.; Menzler, N.H.; Vaßen, R. Self-healing atmospheric plasma sprayed Mn1.0Co1.9Fe0.1O4 protective interconnector coatings for solid oxide fuel cells. J. Power Sources 2017, 363, 185–192. [Google Scholar] [CrossRef]
- Grünwald, N.; Lhuissier, P.; Salvo, L.; Villanova, J.; Menzler, N.H.; Guillon, O.; Martin, C.L.; Vaßen, R. In situ investigation of atmospheric plasma-sprayed Mn-Co-Fe-O by synchrotron X-ray nano-tomography. J. Mater. Sci. 2020, 55, 12725–12736. [Google Scholar] [CrossRef]
- Menzler, N.H.; Sebold, D.; Guillon, O. Post-test characterization of a solid oxide fuel cell stack operated for more than 30,000 hours: The cell. J. Power Sources 2018, 374, 69–76. [Google Scholar] [CrossRef]
- Solid Oxide Fuel Cells. Available online: https://www.energy.gov/fecm/solid-oxide-fuel-cells#:~:text=The%20specific%20goals%20of%20the%20SOFC%20program%20are%3A,greater%20than%2060%25%20without%20carbon%20capture%20and%20storage (accessed on 13 February 2024).
- Alnegren, P.; Froitzheim, J.; Svensson, J. Degradation of ferritic steel interconnects in SOEC environments. ECS Trans. 2013, 27, 2261–2270. [Google Scholar] [CrossRef]
- Wang, R.F.; Sun, Z.H.; Choi, J.P.; Basu, S.N.; Stevenson, J.W.; Tucker, M.C. Ferritic stainless steel interconnects for protonic ceramic electrochemical cell stacks: Oxidation behavior and protective coatings. Int. J. Hydrogen Energy 2019, 44, 25297–25309. [Google Scholar] [CrossRef]
- Lorenzo, M.M.J.; Kolarik, V.; Kuchenreuther-Hummel, V.; Potschke, M.; Schimanke, D. Oxidation of La-Sr-Mn-coated interconnector alloys for steam electrolysis under pressure in pure oxygen and in pure steam. Oxid. Met. 2017, 88, 279–290. [Google Scholar] [CrossRef]
- Cheng, F.P.; Yu, Y.T.; Lu, Y.; Wang, Z.J.; Ling, Y.H.; Jing, C.; Guan, C.Z.; Wang, J.Q. Performance of CoMnO spinel coating onto 441 SS for SOEC interconnect application. Coatings 2022, 12, 1723. [Google Scholar] [CrossRef]
- Xuan, J.L.; Liu, Y.R.; Xu, L.K.; Bai, S.F.; Xin, Y.L.; Wang, L.; Zhang, G.D.; Su, Y.; Xue, L.L.; Li, L. Investigation of acidity on corrosion behavior and surface properties of SS304 in simulated PEMFC cathode environments. Int. J. Hydrogen Energy 2022, 47, 22938–22951. [Google Scholar] [CrossRef]
- Zheng, X.B.; Ji, H.; Huang, J.O.; Ding, C.X. Plasma sprayed Ti and HA coatings: A comparative study between APS and VPS. Acta Metall. Sin. 2005, 18, 339–344. [Google Scholar]
- Li, N.; Araya, S.S.; Cui, X.T.; Kær, S.K. The effects of cationic impurities on the performance of proton exchange membrane water electrolyzer. J. Power Sources 2020, 473, 228617. [Google Scholar] [CrossRef]
- Li, N.; Araya, S.S.; Kær, S.K. The effect of Fe3+ contamination in feed water on proton exchange membrane electrolyzer performance. Int. J. Hydrogen Energy 2019, 44, 12952–12957. [Google Scholar] [CrossRef]
- Wang, X.Y.; Zhang, L.S.; Li, G.F.; Zhang, G.; Shao, Z.G.; Yi, B.L. The influence of ferric ion contamination on the solid polymer electrolyte water electrolysis performance. Electrochim. Acta 2015, 158, 253–257. [Google Scholar] [CrossRef]
- André, J.; Antoni, L.; Petit, J.P.; De, V.E.; Montani, A. Electrical contact resistance between stainless steel bipolar plate and carbon felt in PEFC: A comprehensive study. Int. J. Hydrogen Energy 2009, 34, 3125–3133. [Google Scholar] [CrossRef]
- Lettenmeier, P.; Wang, R.; Abouatallah, R.; Burggraf, F.; Gago, A.S.; Friedricha, K.A. Coated stainless steel bipolar plates for proton exchange membrane electrolyzers. J. Electrochem. Soc. 2016, 163, F3119–F3124. [Google Scholar] [CrossRef]
- Kellenberger, A.; Vaszilcsin, N.; Duca, D.; Dan, M.L.; Duteanu, N.; Morawietz, T.; Biswas, I.; Ansar, S.A.; Gazdzicki, P.; Wirkert, F.J.; et al. Towards replacing titanium with copper in the bipolar plates for proton exchange membrane water electrolysis. Materials 2022, 15, 1628. [Google Scholar] [CrossRef] [PubMed]
- Kawakita, J.; Kuroda, S.; Fukushima, T.; Katanoda, H.; Matsuo, K.; Fukanuma, H. Dense titanium coatings by modified HVOF spraying. Surf. Coat. Tech. 2006, 201, 1250–1255. [Google Scholar] [CrossRef]
- Liu, R.X.; Jia, Q.; Zhang, B.; Lai, Z.G.; Chen, L. Protective coatings for metal bipolar plates of fuel cells: A review. Int. J. Hydrogen Energy 2022, 47, 22915–22937. [Google Scholar] [CrossRef]
- Madadi, F.; Rezaeian, A.; Edris, H.; Zhiani, M. Improving performance in PEMFC by applying different coatings to metallic bipolar plates. Mater. Chem. Phys. 2019, 238, 121911. [Google Scholar] [CrossRef]
- Rendón-Belmonte, M.; Pérez-Quiroz, J.T.; Terán-Guillén, J.; Porcayo-Calderón, J.; Torres-Acosta, A.; Orozco-Gamboa, G. Evaluation of a Cr3C2(NiCr) coating deposited on s4400 by means of an HVOF process and used for flow plates of PEM fuel. Int. J. Electrochem. Sci. 2012, 7, 1079–1092. [Google Scholar] [CrossRef]
- El-Khatib, K.M.; Helal, M.O.A.; El-Moneim, A.A.; Tawfik, H. Corrosion stability of SUS316L HVOF sprayed coatings as lightweight bipolar plate materials in PEM fuel cells. Anti-Corros. Methods Mater. 2004, 51, 136–142. [Google Scholar] [CrossRef]
- Kim, S.C.; Yamaura, S.I.; Shimizu, Y.; Nakashima, K.; Igarashi, T.; Makino, A.; Inoue, A. Production of Ni65Cr15P16B4 metallic glass-coated bipolar plate for fuel cell by high velocity oxy-fuel (HVOF) spray coating method. Mater. Trans. 2010, 51, 1609–1613. [Google Scholar] [CrossRef]





| Coating Method for BPs of SOCs | |||
|---|---|---|---|
| Coating method | Advantages | Disadvantages | Structure feathers |
| Slurry coatings | Simple, low cost | Low efficiency, post-treatment needed | Porous and nonuniform |
| Electrophoretic deposition | Low cost, non-line-of-sight process | Post-treatment needed | Porous |
| Physical vapor deposition | Dense coating | Time-consuming for a thick coating, high vacuum condition, unsuitable for mass production, post-treatment needed | Dense and uniform |
| Electrodeposition | Lost cost, uniform coating thickness | Difficulty for binary or ternary alloy deposition, post-treatment needed | Dense and uniform |
| Atmospheric plasma spraying | Simple, cost-effective, suitable for mass production | Dependent on line-of-sight, some porosities and micro-cracks in the coating | Dense |
| Coating method for BPs of PEMFCs and PEMECs | |||
| Metal nitriding | Coating with low incidence of pinhole defects | High-temperature operation | Dense and uniform |
| Physical vapor deposition | Coating with good adherence | BP size limited by vacuum chamber, pitting corrosion, high vacuum condition | Dense and uniform |
| Chemical vapor deposition | Suitable for mass production, coating with good corrosion resistance | High-temperature operation, high vacuum condition | Dense and uniform |
| Electroplating | Coating with uniform thickness | Suitable for conductive materials, poor adherence | Dense and uniform |
| Vacuum plasma spraying | high deposition rate, thick film | micro-cracks and open pores, dependent on line-of-sight, high vacuum condition | Dense |
| High-velocity oxygen fuel spraying | High deposition rate, thick film | Dependent on line-of-sight, suitable for alloy or non-oxide ceramic materials with low melting point | Dense |
| Atmospheric Plasma Spraying | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Powder | Torch | Ar /L·min−1 | H2 /L·min−1 | I /A | Power /kW | Particle Velocity/m·s−1 | Particle Temperature /°C | Conductivity /S·cm−1 | Thickness of Thermally Grown Oxide/μm | Ref. | |
| LSM | F4 8 mm | 35 | 10 | 650 | 42.9 | 139 | 2276 | 6 | 30 | [46] | |
| 60 | 6 | 700 | 47.3 | 198 | 2425 | 17 | 2 | ||||
| 60 | 3 | 400 | 23.5 | 171 | 2192 | 38 | 2 | ||||
| F4 6 mm | 60 | 3 | 550 | 29.7 | 242 | 2137 | 43 | 2 | |||
| SG100 | 90 | 35 (He) | 590 | 39.2 | 350 | 1725 | 55 | 2 | |||
| MCO | F4 8 m | 35 | 10 | 650 | 42.9 | 127 | 2281 | 11 | 52 | [75] | |
| 60 | 3 | 400 | 23.5 | 154 | 2087 | 19 | 36 | ||||
| 60 | 6 | 700 | 47.3 | 212 | 2318 | 7 | 1 | ||||
| F4 6 mm | 60 | 3 | 550 | 29.7 | 345 | 2251 | 22 | 6 | |||
| SG100 | 90 | 35 (He) | 590 | 39.4 | 580 | 2360 | 38 | 1 | |||
| Vacuum plasma spraying | |||||||||||
| Powder | Plasma enthalpy (MJ·kg−1) | Leak rate (mbar·cm−2 s−1) | Ref. | ||||||||
| Ti | 14.62 | 12.6 | [34] | ||||||||
| 14.66 | 10.5 | ||||||||||
| 21.27 | 4.3 | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Tao, Y.; Wang, Y.; Wu, M.; Zhang, J.; Yu, Y.; Wang, X.; Shao, J. Thermal Sprayed Protective Coatings for Bipolar Plates of Hydrogen Fuel Cells and Water Electrolysis Cells. Coatings 2024, 14, 307. https://doi.org/10.3390/coatings14030307
Liu T, Tao Y, Wang Y, Wu M, Zhang J, Yu Y, Wang X, Shao J. Thermal Sprayed Protective Coatings for Bipolar Plates of Hydrogen Fuel Cells and Water Electrolysis Cells. Coatings. 2024; 14(3):307. https://doi.org/10.3390/coatings14030307
Chicago/Turabian StyleLiu, Tao, Youkun Tao, Yanli Wang, Mingfeng Wu, Jin Zhang, Yang Yu, Xingfu Wang, and Jing Shao. 2024. "Thermal Sprayed Protective Coatings for Bipolar Plates of Hydrogen Fuel Cells and Water Electrolysis Cells" Coatings 14, no. 3: 307. https://doi.org/10.3390/coatings14030307
APA StyleLiu, T., Tao, Y., Wang, Y., Wu, M., Zhang, J., Yu, Y., Wang, X., & Shao, J. (2024). Thermal Sprayed Protective Coatings for Bipolar Plates of Hydrogen Fuel Cells and Water Electrolysis Cells. Coatings, 14(3), 307. https://doi.org/10.3390/coatings14030307