Evolution of Calcareous Deposits and Passive Film on 304 Stainless Steel with Cathodic Polarization in Sea Water
Abstract
:1. Introduction
2. Experimental
2.1. Electrode Preparation
2.2. Test Solution
2.3. Electrochemical Tests
2.4. SEM and XRD Analysis
3. Results and Discussion
3.1. Polarization Tests
3.2. EIS Measurement
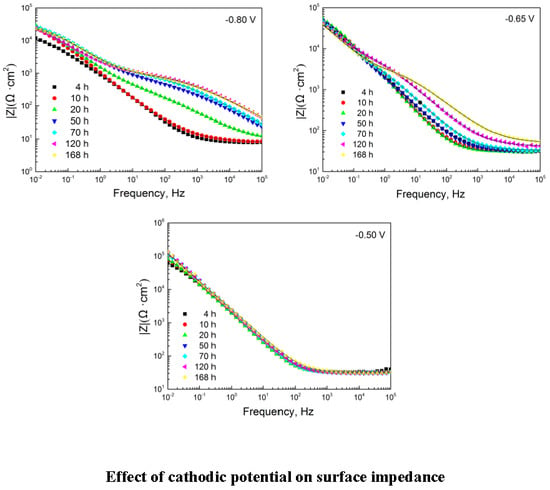
3.2.1. EIS Evolution of 304 SS with Polarization at −0.80 V
3.2.2. EIS Evolution of 304 SS with Polarization at −0.65 V
3.2.3. EIS Evolution of 304 SS with Polarization at −0.50 V
3.3. Surface Analysis
3.3.1. SEM Analysis
3.3.2. EDX Analysis
3.3.3. XRD Analysis
3.4. Discussion
4. Conclusions
- Type 304 SS can be protected effectively from corrosion with cathodic polarization at all the tested potentials. The current density needed for keeping the polarization at −0.80 V vs. SCE was smaller than that for maintaining the polarization at −0.65 V vs. SCE, in relation to the formation of more compact calcareous deposits and the higher resistance of the passive film. This investigation suggests that among the tested potentials, the optimal one for cathodic protection of 304 SS in sea water is −0.50 V vs. SCE, especially for moving parts, with a compromise among the effects over the passive film, calcareous deposits, and protective current density.
- The analyses by EIS, SEM, EDX and XRD demonstrated that calcareous deposits were formed on 304 SS at −0.80 V vs. SCE and −0.65 V vs. SCE, not at −0.50 V vs. SCE. A longer polarization was needed to produce calcareous deposits at −0.65 V vs. SCE than that at −0.80 V vs. SCE. The deposits formed at −0.80 V vs. SCE consisted of CaCO3 predominantly and a small amount of Mg-containing substances, while the precipitates produced at −0.65 V vs. SCE contained only CaCO3. The CaCO3 phase was aragonite.
- With polarization at −0.80 V vs. SCE and −0.65 V vs. SCE, the resistance of passive film on 304 SS decreased initially and then increased, in relation to the reduction of the oxide film and its successive repair. For the stainless steel with polarization at −0.50 V vs. SCE, the film resistance increased with polarization time, indicating that the oxide film is not reduced at this potential. The dominant cathodic reaction at −0.50 V vs. SCE is the oxygen reduction, which elevates the pH value adjacent to the surface, promoting the growth of the oxide film.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lorenzi, S.; Pastore, T.; Bellezze, T.; Fratesi, R. Cathodic protection modelling of a propeller shaft. Corros. Sci. 2016, 108, 36–46. [Google Scholar] [CrossRef]
- Dong, C.F.; Luo, H.; Xiao, K.; Sun, T.; Liu, Q.; Li, X.G. Effect of temperature and Cl− concentration on pitting of 2205 duplex stainless steel. J. Wuhan Univ. Technol. 2011, 26, 641–647. [Google Scholar] [CrossRef]
- Wigen, S.W.; Osvoll, H.; Gartland, P.O.; Huang, W.J. Efficient cathodic protection of stainless steel small bore tubing. In Proceedings of the Corrosion 2007, Nashville, TN, USA, 11–15 March 2007. Paper No. 07078. [Google Scholar]
- Zhang, Y.; Wang, J.Z.; Yin, X.Y.; Yan, F.Y. Tribocorrosion behaviour of 304 stainless steel in different corrosive solutions. Mater. Corros. 2016, 67, 769–777. [Google Scholar] [CrossRef]
- Yang, Y.F.; Scantlebury, J.D.; Koroleva, E.V. A study of calcareous deposits on cathodically protected mild steel in artificial seawater. Metals 2015, 5, 439–456. [Google Scholar] [CrossRef]
- Mancia, F.; Tamba, A. Slow strain rate stress corrosion cracking of AISI 304 Stainless Steel in NaCl solution and its prevention by controlled cathodic protection. Corrosion 1986, 42, 362–367. [Google Scholar] [CrossRef]
- Eashwar, M.; Kumar, P.S.; Ravishankar, R.G. Subramanian Sunlight enhances calcareous deposition on cathodic stainless steel in natural seawater. Biofouling 2013, 29, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Orfei, L.H.; Simison, S.; Busalmen, J.P. Stainless steels can be cathodically protected using energy stored at the marine sediment/seawater interface. Environ. Sci. Technol. 2006, 40, 6473–6478. [Google Scholar] [CrossRef] [PubMed]
- Zucchi, F.; Grassi, V.; Monticelli, C.; Trabanelli, G. Hydrogen embrittlement of duplex stainless steel under cathodic protection in acidic artificial sea water in the presence of sulphide ions. Corros. Sci. 2006, 48, 522–530. [Google Scholar] [CrossRef]
- BS EN 12954 Cathodic Protection of Buried or Immersed Metallic Structures—General Principles and Application for Pipelines; British Standards Institution: London, UK, 2011.
- Heselmans, J.; Buijs, N.W.; Isaac, E. Sacrificial anodes for protection of seawater pump caissons against galvanic corrosion. In Proceedings of the Corrosion 2011, Houston, TX, USA, 13–17 March 2011. Paper No. 11056. [Google Scholar]
- Collazo, A.; Izquierdo, M.; Novoa, X.R.; Perez, C. Surface treatment of carbon steel substrates to prevent cathodic delamination. Electrochim. Acta 2007, 52, 7513–7518. [Google Scholar] [CrossRef]
- Yao, J.Z.; Dong, C.F.; Man, C.; Xiao, K.; Li, X.G. The electrochemical behavior and characteristics of passive film on 2205 duplex stainless steel under various hydrogen charging conditions. Corrosion 2016, 72, 42–50. [Google Scholar] [CrossRef]
- Addari, D.; Elsener, B.; Rossi, A. Electrochemistry and surface chemistry of stainless steels in alkaline media simulating concrete pore solutions. Electrochim. Acta 2008, 53, 8078–8086. [Google Scholar] [CrossRef]
- Bozec, N.L.; Compère, C.; Her, M.L.; Laouenan, A.; Costa, D.; Marcus, P. Influence of stainless steel surface treatment on the oxygen reduction reaction in seawater. Corros. Sci. 2001, 43, 765–786. [Google Scholar] [CrossRef]
- Freire, L.; Carmezim, M.J.; Ferreira, M.G.S.; Montemor, M.F. The electrochemical behaviour of stainless steel AISI 304 in alkaline solutions with different pH in the presence of chlorides. Electrochim. Acta 2011, 56, 5280–5289. [Google Scholar] [CrossRef]
- Burkhard, H.; Kirchheim, R. Dissolution rates of iron and chromium and FeCr alloys in the passive state. Corros. Sci. 1990, 31, 533–538. [Google Scholar]
- Moller, H. The influence of Mg2+ on the formation of calcareous deposits on a freely corroding low carbon steel in seawater. Corros. Sci. 2007, 49, 1992–2001. [Google Scholar] [CrossRef]
- Rousseau, C.; Baraud, F.; Leleyter, L.; Jeannin, M.; Gil, O. Kaolinite influence on calcareous deposit formation. Electrochim. Acta 2009, 55, 196–203. [Google Scholar] [CrossRef]
- Rousseau, C.; Baraud, F.; Leleyter, L.; Jeannin, M.; Gil, O. Calcareous deposit formed under cathodic protection in the presence of natural marine sediments: A 12 months experiment. Corros. Sci. 2010, 52, 2206–2218. [Google Scholar] [CrossRef]
- Sun, W.; Liu, G.C.; Wang, L.D.; Li, Y. A mathematical model for modeling the formation of calcareous deposits on cathodically protected steel in seawater. Electrochim. Acta 2012, 78, 597–608. [Google Scholar] [CrossRef]
- Barchiche, C.; Deslouis, C.; Festy, D.; Gil, O.; Refait, Ph.; Touzain, S.; Tribollet, B. Characterization of calcareous deposits in artificial seawater by impedance techniques: 3-Deposit of CaCO3 in the presence of Mg(II). Electrochim. Acta 2003, 48, 1645–1654. [Google Scholar] [CrossRef]
- Solis, J.L.; Genesca, J. Influence of calcareous deposits on galvanic CP in seawater. Mater. Perform. 2011, 50, 34–38. [Google Scholar]
- Carre, P.; Gunkel-Grillon, P.; Serres, A.; Jeannin, M.; Sabot, R.; Quiniou, T. Calcareous electrochemical precipitation, a new method to trap nickel in seawater. Environ. Chem. Lett. 2017, 15, 151–156. [Google Scholar] [CrossRef]
- Hoseinieh, S.M.; Shahrabi, T.; Ramezanzadeh, B.; Rad, M.F. Influence of sweet crude oil on nucleation and corrosion resistance of calcareous deposits. J. Mater. Eng. Perform. 2016, 25, 4805–4811. [Google Scholar] [CrossRef]
- Sarlak, M.; Shahrab, T.; Zmanzada, M. Investigation of calcareous deposits formation on copper and 316L stainless steel under cathodic polarization in artificial seawater. Prot. Met. Phys. Chem. Surf. 2009, 45, 216–222. [Google Scholar] [CrossRef]
- Smith, S.W. Analysis of the cathodic behavior of aluminum in nature sea water by surface chemistry. Corrosion 1981, 37, 105–121. [Google Scholar]
- Yan, J.F.; White, R.E.; Griffin, R.B. Parametric studies of the formation of calcareous deposits on cathodically protected steel in seawater. J. Electrochem. Soc. 1993, 140, 1275–1280. [Google Scholar] [CrossRef]
- Hartt, W.H.; Culberson, C.H.; Smith, S.W. Calcareous deposits on metal surfaces in seawater—A critical review. Corrosion 1984, 40, 609–618. [Google Scholar] [CrossRef]
- Barchiche, Ch.; Deslouis, C.; Gil, O.; Refait, Ph.; Tribollet, B. Characterisation of calcareous deposits by electrochemical methods: Role of sulphates, calcium concentration and temperature. Electrochim. Acta 2004, 49, 2833–2839. [Google Scholar] [CrossRef]
- Eashwar, M.; Subramanian, G.; Palanichamy, S.; Rajagopal, G.; Madhu, S.; Kamaraj, P. Cathodic behaviour of stainless steel in coastal Indian seawater: Calcareous deposits overwhelm biofilms. Biofouling 2009, 25, 191–201. [Google Scholar] [CrossRef] [PubMed]
- De Saravia, S.G.G.; de Mele, M.F.L.; Videla, H.A.; Edyvean, R.G.J. Bacterial biofilms on cathodically protected stainless steel. Biofouling 1997, 11, 1–17. [Google Scholar] [CrossRef]
- Liu, C.T.; Wu, J.K. Influence of pH on the passivation behaviour of 254 SMO stainless steel in 3.5% NaCl solution. Corros. Sci. 2007, 49, 2198–2209. [Google Scholar] [CrossRef]
- Liu, X.H.; Han, E.H.; Wu, X.Q. Effects of pH value on characteristics of oxide films on 316L stainless steel in Zn-injected borated and lithiated high temperature water. Corros. Sci. 2014, 78, 200–207. [Google Scholar] [CrossRef]
- Luo, H.; Dong, C.F.; Li, X.G.; Xiao, K. The electrochemical behaviour of 2205 duplex stainless steel in alkaline solutions with different pH in the presence of chloride. Electrochim. Acta 2012, 64, 211–220. [Google Scholar] [CrossRef]
- Luo, H.; Su, H.Z.; Dong, C.F.; Li, X.G. Passivation and electrochemical behavior of 316L stainless steel in chlorinated simulated concrete pore solution. Appl. Surf. Sci. 2017, 400, 38–48. [Google Scholar] [CrossRef]
- Ramasubramanian, N.; Preocanin, N.; Davidson, R.D. Analysis of passive films on stainless steel by cyclic voltammetry and auger spectroscopy. J. Electrochem. Soc. 1985, 16, 793–798. [Google Scholar] [CrossRef]
- Karoui, H.; Riffault, B.; Jeannin, M.; Kahoul, A.; Gil, O.; Amor, M.B.; Tlili, M.M. Electrochemical scaling of stainless steel in artificial seawater: Role of experimental conditions on CaCO3 and Mg(OH)2 formation. Desalination 2013, 311, 234–240. [Google Scholar] [CrossRef]
- Neville, A.; Morizot, A.P. Calcareous scales formed by cathodic protection—An assessment of characteristics and kinetics. J. Cryst. Growth 2002, 243, 490–502. [Google Scholar] [CrossRef]
- Mohammadi, F.; Nickchi, T.; Attar, M.M.; Alfantazi, A. EIS study of potentiostatically formed passive film on 304 stainless steel. Electrochim. Acta 2011, 56, 8727–8733. [Google Scholar] [CrossRef]
- Ge, H.H.; Zhou, G.D.; Wu, W.Q. Passivation model of 316L stainless steel in simulated cooling water and the effect of sulfide on the passive film. Appl. Surf. Sci. 2003, 211, 321–334. [Google Scholar] [CrossRef]
- Metikoš-Huković, M.; Babić, R.; Grubač, Z.; Petrovićet, Z.; Lajçi, N. High corrosion resistance of austenitic stainless steel alloyed with nitrogen in an acid solution. Corros. Sci. 2011, 53, 2176–2183. [Google Scholar] [CrossRef]
- Hirschorn, B.; Orazem, M.E.; Tribollet, B.; Vivier, V.; Frateur, I.; Musiani, M. Determination of effective capacitance and film thickness from constant-phase-element parameters. Electrochim. Acta 2010, 55, 6218–6227. [Google Scholar] [CrossRef]
- Lewandowski, Z.; Lee, W.C.; Characklis, W.G.; Little, B.J. Dissolved oxygen and pH microelectrode measurements at water-immersed metal surfaces. Corrosion 1989, 45, 92–98. [Google Scholar] [CrossRef]
- Rondelli, G.; Torricelli, P.; Fini, M.; Giardino, R. In vitro corrosion study by EIS of a nickel-free stainless steel for orthopaedic applications. Biomaterials 2005, 26, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, A.I.; Julián, L.C. Influence of electrochemical potential on the tribocorrosion behaviour of high carbon CoCrMo biomedical alloy in simulated body fluids by electrochemical impedance spectroscopy. Electrochim. Acta 2010, 55, 5428–5439. [Google Scholar] [CrossRef]
- Blanco, G.; Bautista, A.; Takenouti, H. EIS study of passivation of austenitic and duplex stainless steels reinforcements in simulated pore solutions. Cem. Concr. Compos. 2006, 28, 212–219. [Google Scholar] [CrossRef]
- Veleva, L.; Alpuche-Aviles, M.A.; Graves-Brook, M.K.; Wipf, D.O. Comparative cyclic voltammetry and surface analysis of passive films grown on stainless steel 316 in concrete pore model solutions. J. Electroanal. Chem. 2002, 537, 85–93. [Google Scholar] [CrossRef]
- Bautista, A.; Blanco, G.; Velasco, F.; Gutiérrez, A.; Soriano, L.; Palomares, F.J.; Takenouti, H. Changes in the passive layer of corrugated austenitic stainless steel of low nickel content due to exposure to simulated pore solutions. Corros. Sci. 2009, 51, 785–792. [Google Scholar] [CrossRef]
- Freire, L.; Carmezim, M.J.; Ferreira, M.G.S.; Montemor, M.F. The passive behaviour of AISI 316 in alkaline media and the effect of pH: A combined electrochemical and analytical study. Electrochim. Acta 2010, 55, 6174–6181. [Google Scholar] [CrossRef]
- Freire, L.; Catarino, M.A.; Godinho, M.I.; Ferreira, M.J.; Ferreira, M.G.S.; Simões, A.M.P.; Montemor, M.F. Electrochemical and analytical investigation of passive films formed on stainless steels in alkaline media. Cem. Concr. Compos. 2012, 34, 1075–1081. [Google Scholar] [CrossRef]
- ISO 15589-2 Petroleum, Petrochemical and Natural Gas Industries—Cathodic Protection of Pipeline Transportation Systems—Part 2: Offshore Pipelines; International Organization for Standardization: Vernier, Geneva, Switzerland, 2012.











| t (h) | Rs (Ω·cm2) | Qdl (μF·cm2) | n1 | Rct (kΩ·cm2) | Qc (μF·cm2) | n2 | Rc (Ω·cm2) | Qox (μF·cm2) | n3 | Rox (Ω·cm2) |
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 7.773 | 52.49 | 0.854 | 199.0 | – | – | – | 469.2 | 0.768 | 463.9 |
| 4 | 7.833 | 326.1 | 0.736 | 14.48 | – | – | – | 759.3 | 0.918 | 196.1 |
| 10 | 7.924 | 226.6 | 0.781 | 34.45 | – | – | – | 304.7 | 0.488 | 15.28 |
| 20 | 8.780 | 104.4 | 0.905 | 42.29 | 294.9 | 0.475 | 2459 | 102.9 | 0.575 | 363.7 |
| 50 | 3.617 | 172.5 | 0.587 | 48.32 | 443.3 | 0.999 | 4495 | 34.79 | 0.545 | 489.6 |
| 70 | 2.736 | 184.8 | 0.577 | 60.53 | 392.4 | 0.940 | 5731 | 27.22 | 0.545 | 797.2 |
| 120 | 1.016 | 173.5 | 0.641 | 58.54 | 313.2 | 0.652 | 4761 | 18.69 | 0.598 | 634.8 |
| 168 | 2.933 | 229.8 | 0.517 | 130.6 | 323.5 | 0.924 | 7290 | 18.25 | 0.537 | 725.6 |
| t (h) | Rs (Ω·cm2) | Qdl (μF·cm2) | n1 | Rct (kΩ·cm2) | Qc (μF·cm2) | n2 | Rc (Ω·cm2) | Qox (μF·cm2) | n3 | Rox (Ω·cm2) |
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 27.77 | 107.5 | 0.819 | 105.1 | – | – | – | 69.76 | 0.840 | 198.6 |
| 4 | 30.80 | 102.4 | 0.776 | 76.61 | – | – | – | 585.8 | 0.622 | 42.14 |
| 10 | 31.00 | 123.5 | 0.799 | 59.58 | – | – | – | 223.5 | 0.815 | 4.697 |
| 20 | 30.72 | 149.9 | 0.785 | 26.62 | – | – | – | 386.2 | 0.998 | 5.306 |
| 50 | 31.17 | 155.2 | 0.789 | 146.9 | 289.1 | 0.707 | 911.4 | 31.32 | 0.998 | 1.483 |
| 70 | 32.49 | 172.2 | 0.761 | 189.6 | 145.3 | 0.706 | 2261 | 8.164 | 0.993 | 3.329 |
| 120 | 38.02 | 163.8 | 0.678 | 281.8 | 69.34 | 0.696 | 3675 | 23.34 | 0.730 | 9.879 |
| 168 | 29.97 | 159.1 | 0.637 | 281.4 | 35.13 | 0.655 | 2861 | 5.073 | 0.572 | 37.76 |
| t (h) | Rs (Ω·cm2) | Qdl (μF·cm2) | n1 | Rct (kΩ·cm2) | Qox (μF·cm2) | n3 | Rox (Ω·cm2) |
|---|---|---|---|---|---|---|---|
| 0 | 28.14 | 31.75 | 0.768 | 29.86 | 84.23 | 0.848 | 168.9 |
| 4 | 34.18 | 112.7 | 0.885 | 78.12 | 645.8 | 0.869 | 222.8 |
| 10 | 31.28 | 183.9 | 0.830 | 88.54 | 192.2 | 0.980 | 2306 |
| 20 | 30.79 | 205.4 | 0.998 | 95.18 | 232.5 | 0.810 | 17420 |
| 50 | 31.21 | 140.8 | 0.998 | 181.5 | 221.3 | 0.783 | 20640 |
| 70 | 31.21 | 159.5 | 0.999 | 219.4 | 175.2 | 0.820 | 32060 |
| 120 | 30.41 | 126.1 | 0.974 | 335.9 | 236.2 | 0.773 | 32800 |
| 168 | 31.51 | 124.5 | 0.989 | 286.6 | 206.0 | 0736 | 22510 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, T.; Huang, G.; Lv, P.; Xu, L.; Ma, L. Evolution of Calcareous Deposits and Passive Film on 304 Stainless Steel with Cathodic Polarization in Sea Water. Coatings 2018, 8, 194. https://doi.org/10.3390/coatings8050194
Sun T, Huang G, Lv P, Xu L, Ma L. Evolution of Calcareous Deposits and Passive Film on 304 Stainless Steel with Cathodic Polarization in Sea Water. Coatings. 2018; 8(5):194. https://doi.org/10.3390/coatings8050194
Chicago/Turabian StyleSun, Tianxiang, Guosheng Huang, Ping Lv, Likun Xu, and Li Ma. 2018. "Evolution of Calcareous Deposits and Passive Film on 304 Stainless Steel with Cathodic Polarization in Sea Water" Coatings 8, no. 5: 194. https://doi.org/10.3390/coatings8050194
APA StyleSun, T., Huang, G., Lv, P., Xu, L., & Ma, L. (2018). Evolution of Calcareous Deposits and Passive Film on 304 Stainless Steel with Cathodic Polarization in Sea Water. Coatings, 8(5), 194. https://doi.org/10.3390/coatings8050194