Corrosion Protection of N80 Steel in Hydrochloric Acid Medium Using Mixed C15H15NO and Na2WO4 Inhibitors
Abstract
:1. Introduction
2. Experimental Procedures
3. Results and Discussion
3.1. IR Spectrum Analysis of C15H15NO
3.2. Electrochemical Analysis
3.2.1. Potentiodynamic Polarization Studies
3.2.2. EIS Studies
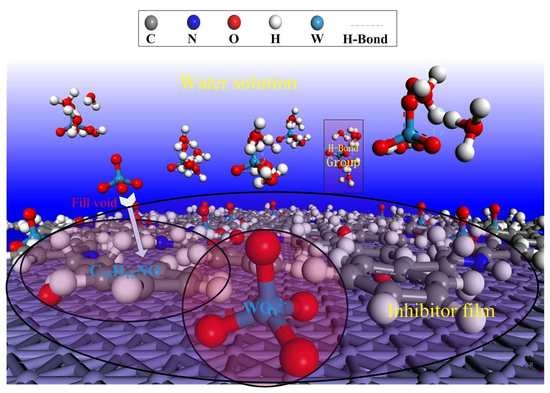
3.3. Analysis of Scanning Electron Microscopy
3.4. X-ray Photoelectron Spectroscopy
3.4.1. XPS Survey Spectra Studies
3.4.2. Analysis of Binding Energy of Film
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Quraishi, M.A.; Sardar, R. Corrosion inhibition of mild steel in acid solutions by some aromatic oxadiazoles. Mater. Chem. Phys. 2003, 78, 425–431. [Google Scholar] [CrossRef]
- El-Maksoud, S.A.; Fouda, A. Some pyridine derivatives as corrosion inhibitors for carbon steel in acidic medium. Mater. Chem. Phys. 2005, 93, 84–90. [Google Scholar] [CrossRef]
- Migahed, M.; Nassar, I. Corrosion inhibition of tubing steel during acidization of oil and gas wells. Electrochim. Acta 2008, 53, 2877–2882. [Google Scholar] [CrossRef]
- Finšgar, M.; Jackson, J. Application of corrosion inhibitors for steels in acidic media for the oil and gas industry: A review. Corros. Sci. 2014, 86, 17–41. [Google Scholar] [CrossRef]
- Yadav, M.; Behera, D.; Sharma, U. Corrosion protection of N80 steel in hydrochloric acid by substituted amino acids. Corros. Eng. Sci. Technol. 2013, 48, 19–27. [Google Scholar] [CrossRef]
- Yadav, M.; Sharma, U. Eco-friendly corrosion inhibitors for N80 steel in hydrochloric acid. J. Mater. Environ. Sci. 2011, 2, 407–414. [Google Scholar]
- Kumar, S.; Sharma, D.; Yadav, P.N.; Yadav, M. Experimental and quantum chemical studies on corrosion inhibition effect of synthesized organic compounds on N80 steel in hydrochloric acid. Ind. Eng. Chem. Res. 2013, 52, 14019–14029. [Google Scholar] [CrossRef]
- Vishwanatham, S.; Sinha, P. Corrosion protection of N80 steel in HCL by condensation products of aniline and phenol. Anti-Corros. Methods Mater. 2009, 56, 139–144. [Google Scholar] [CrossRef]
- Emranuzzaman; Kumar, T.; Vishwanatham, S.; Udayabhanu, G. Synergistic effects of formaldehyde and alcoholic extract of plant leaves for protection of N80 steel in 15% HCL. Corros. Eng. Sci. Technol. 2004, 39, 327–332. [Google Scholar] [CrossRef]
- Gardner, G.S.; Saukaitis, A.J. Inhibitor Acid. U.S. Patent 2,807,585, 2 December 1957. [Google Scholar]
- Quraishi, M.A.; Ahamad, I.; Singh, A.K.; Shukla, S.K.; Lal, B.; Singh, V. N-(piperidinomethyl)-3-[(pyridylidene) amino]isatin: A new and effective acid corrosion inhibitor for mild steel. Mater. Chem. Phys. 2008, 112, 1035–1039. [Google Scholar] [CrossRef]
- Olivares-Xometl, O.; Likhanova, N.; Domínguez-Aguilar, M.; Arce, E.; Dorantes, H.; Arellanes-Lozada, P. Synthesis and corrosion inhibition of α-amino acids alkylamides for mild steel in acidic environment. Mater. Chem. Phys. 2008, 110, 344–351. [Google Scholar] [CrossRef]
- Quartarone, G.; Battilana, M.; Bonaldo, L.; Tortato, T. Investigation of the inhibition effect of indole-3-carboxylic acid on the copper corrosion in 0.5 M H2SO4. Corros. Sci. 2008, 50, 3467–3474. [Google Scholar] [CrossRef]
- Ebenso, E.E.; Okafor, P.C.; Ekpe, U.J. Studies on the inhibition of aluminium corrosion by 2-acetylphenothiazine in chloroacetic acids. Anti-Corros. Methods Mater. 2003, 50, 414–421. [Google Scholar] [CrossRef]
- Meng, F.N.; Li, Q.D.; Li, S.J. Synergistic inhibition mechanism of mannich bases and thiourea in corrosion system of gas-field wastewater. Surf. Technol. 2014, 43, 90–93. [Google Scholar]
- Shibli, S.M.A.; Saji, V.S. Co-inhibition characteristics of sodium tungstate with potassium iodate on mild steel corrosion. Corros. Sci. 2005, 47, 2213–2224. [Google Scholar] [CrossRef]
- Huang, L.; Xu, X.-E.; Wang, W.Q. Corrosion inhibition performances of sodium tungstate and its composite for carbon steel in simulated seawater. Surf. Technol. 2014, 43, 25–29. (In Chinese) [Google Scholar]
- Ren, X.G.; Zhou, J.M.; Liu, D.; Li, L.; Song, Y.J. Corrosion inhibition of mannich base corrosion inhibitor to N80 steel. Drill. Fluid Complet. Fluid 2010, 27, 72–73. (In Chinese) [Google Scholar]
- Hu, J.; Wang, Y.; Yu, L.J.; Zou, Y.Q.; Wang, Y.Q. An Investigation of a combined thiourea and hexamethylenetetramine as inhibitors for corrosion of N80 in 15% HCl solution: Electrochemical experiments and quantum chemical calculation. Int. J. Corros. 2015, 2015. [Google Scholar] [CrossRef]
- El-Moneim, A.A.; Akiyama, E.; Habazaki, H.; Kawashima, A.; Asami, K.; Hashimotoetc, K. XPS and electrochemical studies on the corrosion behavior of sputter-deposited amorphous Mn-Nb alloys in a neutral chloride solution. Corros. Sci. 1998, 40, 1513–1531. [Google Scholar] [CrossRef]
- Geler, E.; Azambuja, D.S. Corrosion inhibition of copper in chloride solutions by pyrazole. Corros. Sci. 2000, 42, 631–643. [Google Scholar] [CrossRef]
- Epelboin, I.; Keddam, M.; Mattos, O.R.; Takenouti, H. The dissolution and passivation of Fe and Fe-Cr alloys in acidified sulphate medium: Influences of pH and Cr content. Corros. Sci. 1979, 19, 1105–1112. [Google Scholar] [CrossRef]
- Epelboin, I.; Keddam, M. Kinetics of formation of primary and Secondary passivity in sulphuric aqueous media. Electrochim. Acta 1972, 17, 177–186. [Google Scholar] [CrossRef]
- Ma, H.; Chen, S.; Niu, L.; Li, S.; Li, D. Inhibition of copper corrosion by several Schiff bases in aerated halide solutions. J. Appl. Electrochem. 2002, 32, 65–72. [Google Scholar] [CrossRef]
- Gueshi, T.; Tokuda, K.; Matsuda, H. Voltammetry at partially covered electrodes: Part I. Chronopotentiometry and chronoamperometry at model electrodes. J. Electroanal. Chem. Interfacial Electrochem. 1978, 89, 247–260. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, J.; Wang, Y.Q.; Yu, L.J. A New Method for Preventing Corrosion Failure: Thiourea and Hexamethylenetetramine as Inhibitor for Copper. Bull. Korean Chem. Sci. 2016, 37, 1797–1811. [Google Scholar] [CrossRef]
- Asawa, M.; Devasenapathi, A.; Fujisawa, M. Effect of corrosion product layer on SCC susceptibility of copper containing type 304 stainless steel in 1 M H2SO4. Mater. Sci. Eng. A 2004, 366, 292–298. [Google Scholar] [CrossRef]
- Tüken, T.; Erbil, M. The use of polyindole for prevention of copper corrosion. Surf. Coat. Technol. 2006, 200, 4802–4809. [Google Scholar] [CrossRef]
- Hu, S.Q.; Hu, J.C.; Fan, C.C.; Mi, S.Q.; Zhang, J.; Guo, W.Y. Corrosion inhibition of Q235 steel by a novel imidazoline compound under H2S and CO2 coexistence. Acta Phys. Chimica Sin. 2010, 8, 2163–2170. [Google Scholar]
- Luo, H.; Su, H.Z.; Dong, C.F.; Xiao, K.; Li, X.G. Electrochemical and passivation behavior investigation of ferritic stainless steel in alkaline environment. Constr. Build. Mater. 2015, 96, 502–507. [Google Scholar] [CrossRef]
- Zarrok, H.; Zarrouk, A.; Hammouti, B.; Salghi, R.; Jama, C.; Bentiss, F. Corrosion control of carbon steel in phosphoric acid by purpald–weight loss, electrochemical and XPS studies. Corros. Sci. 2012, 64, 243–252. [Google Scholar] [CrossRef]
- Bouanis, M.; Tourabi, M.; Nyassi, A.; Zarrouk, A.; Jama, C.; Bentiss, F. Corrosion inhibition performance of 2,5-bis(4-dimethylaminophenyl)-1,3,4-oxadiazole for carbon steel in HCl solution: Gravimetric, electrochemical and XPS studies. Appl. Surf. Sci. 2016, 389, 952–966. [Google Scholar] [CrossRef]
- Descostes, M.; Mercier, F.; Thromat, N.; Beaucaire, C.; Gautier-Soyer, M. Use of XPS in the determination of chemical environment and a data basis in binding energies for Fe and S reference compounds and applications to the evidence of surface species of an oxidized pyrite in a carbonate medium. Appl. Surf. Sci. 2000, 165, 288–302. [Google Scholar] [CrossRef]
- Feng, Z.C.; Cheng, X.Q.; Dong, C.F.; Xu, L.; Li, X.G. Passivity of 316L stainless steel in borate buffer solution studied by Mott-Schottky analysis, atomic adsorption spectrometry and X-ray photoelectron spectroscopy. Corros. Sci. 2010, 52, 3646–3653. [Google Scholar] [CrossRef]
- Devaux, R.; Vouagner, D.; De Becdelievre, A.M.; Duret-Thual, C. Electrochemical and surface studies of the ageing of passive layers grown on stainless steel in neutral chloride solution. Corros. Sci. 1994, 36, 171–186. [Google Scholar] [CrossRef]
- Nakayama, N.; Obuchi, A. Inhibitory effects of 5-aminouracil on cathodic reactions of steels in saturated Ca(OH)2 solutions. Corros. Sci. 2003, 45, 2075–2092. [Google Scholar] [CrossRef]
- Pech-Canul, M.A.; Bartolo-Perez, P. Inhibition effects of N-phosphono-methyl-glyciney/Zn2+ mixtures on corrosion of steel in neutral chloride solutions. Surf. Coat. Technol. 2004, 184, 133–140. [Google Scholar] [CrossRef]
- Galtayries, A.; Warocquier-Clérout, R.; Nagel, M.D.; Marcus, P. Fibronectin adsorption on Fe-Cr alloy studied by XPS. Surf. Interface Anal. 2006, 38, 186–190. [Google Scholar] [CrossRef]
- Sastri, V.S.; Elboujdaini, M.; Rown, J.R.; Perumareddi, J.R. Surface analysis of inhibitor films formed in hydrogen sulfide medium. Corrosion 1996, 52, 447–452. [Google Scholar] [CrossRef]
- Vishwanatham, S.; Haldar, N. Furfuryl alcohol as corrosion inhibitor for N80 steel in hydrochloric acid. Corros. Sci. 2008, 50, 2999–3004. [Google Scholar] [CrossRef]
- Yadav, M.; Behera, D.; Sharma, U. Nontoxic corrosion inhibitors for N80 steel in hydrochloric acid. Arab. J. Chem. 2012, 4, 1487–1495. [Google Scholar]
- Yadav, M.; Sharma, U.; Yadav, P.N. Isatin compounds as corrosion inhibitors for N80 steel in 15% HCl. Egypt. J. Petroleum. 2013, 22, 335–344. [Google Scholar] [CrossRef]
- Zhou, Y.; Zuo, Y.; Lin, B. The compounded inhibition of sodium molybdate and benzotriazole on pitting corrosion of Q235 steel in NaCl + NaHCO3 solution. Mater. Chem. Phys. 2017, 192, 86–93. [Google Scholar] [CrossRef]
- Bokati, K.S.; Dehghanian, C.; Yari, S. Corrosion inhibition of copper, mild steel and galvanically coupled copper-mild steel in artificial sea water in presence of 1H-benzotriazole, sodium molybdate and sodium phosphate. Corros. Sci. 2017, 126, 272–285. [Google Scholar] [CrossRef]
- Tremont, R.; Jesus-Cardona, H.D.; Garcia-Orozco, J. 3-Mercaptopropyltrimethoxysilane as a Cu corrosion inhibitor in KCl solution. J. Appl. Electrochem. 2000, 30, 737–743. [Google Scholar] [CrossRef]
- Olivares, O.; Likhanova, N.V.; Gomez, B.; Navarrete, J.; Llanos-Serrano, M.E.; Arce, E.; Hallen, J.M. Electrochemical and XPS studies of decylamides of α-amino acids adsorption on carbon steel in acidic environment. Appl. Surf. Sci. 2006, 252, 2894–2909. [Google Scholar] [CrossRef]
- Hosseini, M.; Mertens, S.F.L.; Arshadi, M.R. Synergism and antagonism in mild steel corrosion inhibition by sodium dodecylbenzenesulphonate and hexamethyleneteramine. Corros. Sci. 2003, 45, 1473–1489. [Google Scholar] [CrossRef]








| Sample/mol·L–1 | Rs/Ω·cm2 | Cf/S·sn·cm−2/×10−5 | n1/0 < n < 1 | Rf /Ω·cm2 | Cdl/S·sn·cm−2/*10−2 | n2 /0 < n < 1 | Rct/Ω·cm2 | θ | ||
|---|---|---|---|---|---|---|---|---|---|---|
| HCl | C15H15NO | Na2WO4 | ||||||||
| 4.865 | 0 | 0 | 0.6821 | 2.375 | 0.9656 | 4.102 | 0.057 | 0.6694 | 39.34 | – |
| 0.004 | 0.0002 | 0.8403 | 1.833 | 0.8728 | 3.789 | 0.089 | 0.5647 | 102.1 | 0.615 | |
| 0.002 | 0.0002 | 0.7169 | 2.184 | 0.8899 | 3.064 | 0.046 | 0.6856 | 83.99 | 0.532 | |
| 0.0004 | 0.0002 | 0.7487 | 2.177 | 0.9937 | 2.901 | 0.011 | 0.5495 | 114.8 | 0.657 | |
| 0.00025 | 0.0002 | 0.8093 | 2.169 | 0.8972 | 3.918 | 0.035 | 0.5889 | 92.24 | 0.574 | |
| 0.0002 | 0.0002 | 0.7571 | 2.484 | 0.9844 | 5.217 | 0.034 | 0.6518 | 260.9 | 0.849 | |
| 0.00013 | 0.0002 | 0.8079 | 1.89 | 0.9423 | 5.589 | 0.034 | 0.634 | 139.2 | 0.717 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Wang, T.; Wang, Z.; Wei, L.; Zhu, J.; Zheng, M.; Chen, Z. Corrosion Protection of N80 Steel in Hydrochloric Acid Medium Using Mixed C15H15NO and Na2WO4 Inhibitors. Coatings 2018, 8, 315. https://doi.org/10.3390/coatings8090315
Hu J, Wang T, Wang Z, Wei L, Zhu J, Zheng M, Chen Z. Corrosion Protection of N80 Steel in Hydrochloric Acid Medium Using Mixed C15H15NO and Na2WO4 Inhibitors. Coatings. 2018; 8(9):315. https://doi.org/10.3390/coatings8090315
Chicago/Turabian StyleHu, Jun, Tiantian Wang, Zhen Wang, Liping Wei, Jianbo Zhu, Maosheng Zheng, and Zhong Chen. 2018. "Corrosion Protection of N80 Steel in Hydrochloric Acid Medium Using Mixed C15H15NO and Na2WO4 Inhibitors" Coatings 8, no. 9: 315. https://doi.org/10.3390/coatings8090315
APA StyleHu, J., Wang, T., Wang, Z., Wei, L., Zhu, J., Zheng, M., & Chen, Z. (2018). Corrosion Protection of N80 Steel in Hydrochloric Acid Medium Using Mixed C15H15NO and Na2WO4 Inhibitors. Coatings, 8(9), 315. https://doi.org/10.3390/coatings8090315