Benefits of Nanomedicine for Therapeutic Intervention in Malignant Diseases
Abstract
:1. Introduction
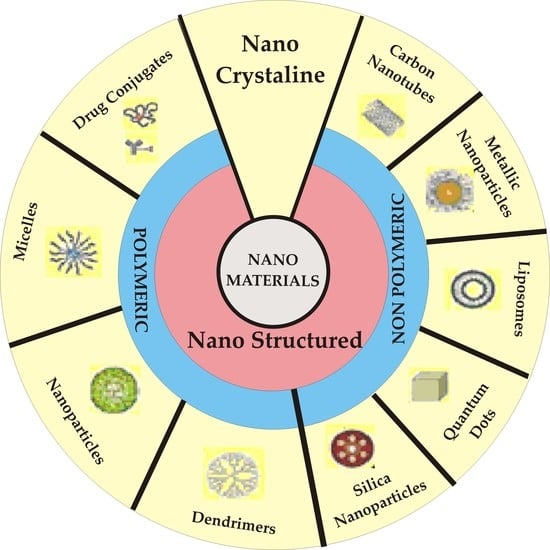
2. Nanotechnology in Cancer Therapy
3. Modeling Bioavailability—A Real Challenge in Oncology Research
4. Achieved Objectives—FDA Approved Nanomedicines Used in Cancer Therapy
5. Limitations
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S. Genomics-guided immunotherapy for precision medicine in cancer. Cancer Biother. Radiopharm. 2019. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Xie, Y.T.; Girnita, L.; Nilsson, G.; Dricu, A.; Wejde, J.; Larsson, O. Regulatory role of mevalonate and N-linked glycosylation in proliferation and expression of the EWS/FLI-1 fusion protein in Ewing’s sarcoma cells. Exp. Cell Res. 1999, 246, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Cosaceanu, D.; Carapancea, M.; Budiu, R.; Martinsson, H.-S.; Starborg, M.; Vrabete, M.; Kanter, L.; Lewensohn, R.; Dricu, A. Comparison of three approaches for inhibiting insulin-like growth factor I receptor and their effects on NSCLC cell lines in vitro. Growth Factors 2007, 25, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Umemoto, S.; Haruta, M.; Sakisaka, M.; Ikeda, T.; Tsukamoto, H.; Komohara, Y.; Takeya, M.; Nishimura, Y.; Senju, S. Cancer therapy with MHC-deficient and interferon β-producing myeloid cells derived from allogeneic embryonic stem cells. Cancer Sci. 2019. [Google Scholar] [CrossRef] [PubMed]
- Carapancea, M.; Alexandru, O.; Fetea, A.S.; Dragutescu, L.; Castro, J.; Georgescu, A.; Popa-Wagner, A.; Backlund, M.L.; Lewensohn, R.; Dricu, A. Growth factor receptors signaling in glioblastoma cells: Therapeutic implications. J. Neurooncol. 2009, 92, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.; Gu, F.X.; Langer, R.; Farokhzad, O.C.; Lippard, S.J. Targeted delivery of cisplatin to prostate cancer cells by aptamer functionalized Pt(IV) prodrug-PLGA-PEG nanoparticles. Proc. Natl. Acad. Sci. USA 2008, 105, 17356–17361. [Google Scholar] [CrossRef] [PubMed]
- Albanese, A.; Tang, P.S.; Chan, W.C. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. J. Control. Release 2015, 200, 138–157. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef]
- Palazzolo, S.; Bayda, S.; Hadla, M.; Caligiuri, I.; Corona, G.; Toffoli, G.; Rizzolio, F. The clinical translation of organic nanomaterials for cancer therapy: A focus on polymeric nanoparticles, micelles, liposomes and exosomes. Curr. Med. Chem. 2018, 25, 4224–4268. [Google Scholar] [CrossRef] [PubMed]
- Bayda, S.; Hadla, M.; Palazzolo, S.; Riello, P.; Corona, G.; Toffoli, G.; Rizzolio, F. Inorganic nanoparticles for cancer therapy: A transition from lab to clinic. Curr. Med. Chem. 2018, 25, 4269–4303. [Google Scholar] [CrossRef] [PubMed]
- Pokropivny, V.V.; Skorokhod, V.V. Classification of nanostructures by dimensionality and concept of surface forms engineering in nanomaterial science. Mater. Sci. Eng. C 2007, 27, 990–993. [Google Scholar] [CrossRef]
- Wagner, S.; Gondikas, A.; Neubauer, E.; Hofmann, T.; von der Kammer, F. Spot the difference: Engineered and natural nanoparticles in the environment—Release, behavior, and fate. Angew. Chem. Int. Ed. 2014, 53, 12398–12419. [Google Scholar] [CrossRef] [PubMed]
- Sanchis, A.; Salvador, J.P.; Marco, M.P. Light-induced mechanisms for nanocarrier’s cargo release. Colloids Surf. B Biointerfaces 2019, 173, 825–832. [Google Scholar] [CrossRef]
- Florence, A.T. “Targeting” nanoparticles: The constraints of physical laws and physical barriers. J. Control. Release 2012, 164, 115–124. [Google Scholar] [CrossRef]
- Nichols, J.W.; Bae, Y.H. Odyssey of a cancer nanoparticle: From injection site to site of action. Nano Today 2012, 7, 606–618. [Google Scholar] [CrossRef] [Green Version]
- Lazarovits, J.; Chen, Y.Y.; Sykes, E.A.; Chan, W.C.W. Nanoparticle–blood interactions: The implications on solid tumour targeting. Chem. Commun. 2015, 51, 2756–2767. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Saraiva, C.; Praca, C.; Ferreira, R.; Santos, T.; Ferreira, L.; Bernardino, L. Nanoparticle-mediated brain drug delivery: Overcoming blood-brain barrier to treat neurodegenerative diseases. J. Control. Release 2016, 235, 34–47. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Ran, F.; Cui, Y.; Liu, C.; Zhao, Q.; Gao, Y.; Wang, D.; Wang, S. A comparison between sphere and rod nanoparticles regarding their in vivo biological behavior and pharmacokinetics. Sci. Rep. 2017, 7, 4131. [Google Scholar] [CrossRef]
- Stylianopoulos, T.; Poh, M.-Z.; Insin, N.; Bawendi, M.G.; Fukumura, D.; Munn, L.L.; Jain, R.K. Diffusion of particles in the extracellular matrix: The effect of repulsive electrostatic interactions. Biophys. J. 2010, 99, 1342–1349. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano–bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Din, F.U.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.X.; Ahmed, T.; Li, L.Y.; Li, J.; Abbasi, A.Z.; Wu, X.Y. Design of nanocarriers for nanoscale drug delivery to enhance cancer treatment using hybrid polymer and lipid building blocks. Nanoscale 2017, 9, 1334–1355. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, P.L.; Harada, T.; Christian, D.A.; Pantano, D.A.; Tsai, R.K.; Discher, D.E. Minimal “Self” peptides that inhibit phagocytic clearance and enhance delivery of nanoparticles. Science 2013, 339, 971–975. [Google Scholar] [CrossRef] [PubMed]
- Nie, S. Understanding and overcoming major barriers in cancer nanomedicine. Nanomedicine 2010, 5, 523–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- von Roemeling, C.; Jiang, W.; Chan, C.K.; Weissman, I.L.; Kim, B.Y. Breaking down the barriers to precision cancer nanomedicine. Trends Biotechnol. 2017, 35, 159–171. [Google Scholar] [CrossRef]
- Jackson, M.A.; Werfel, T.A.; Curvino, E.J.; Yu, F.; Kavanaugh, T.E.; Sarett, S.M.; Dockery, M.D.; Kilchrist, K.V.; Jackson, A.N.; Giorgio, T.D.; et al. Zwitterionic nanocarrier surface chemistry improves siRNA tumor delivery and silencing activity relative to polyethylene glycol. ACS Nano 2017, 11, 5680–5696. [Google Scholar] [CrossRef]
- Fang, R.H.; Kroll, A.V.; Gao, W.; Zhang, L. Cell membrane coating nanotechnology. Adv. Mater. 2018, 30, e1706759. [Google Scholar] [CrossRef]
- Duan, X.; Li, Y. Physicochemical characteristics of nanoparticles affect circulation, biodistribution, cellular internalization, and trafficking. Small 2013, 9, 1521–1532. [Google Scholar] [CrossRef] [PubMed]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, A.; Villa, C.H.; Bander, E.; Rey, D.A.; Bergkvist, M.; Batt, C.A.; Manova-Todorova, K.; Deen, W.M.; Scheinberg, D.A.; McDevitt, M.R. Paradoxical glomerular filtration of carbon nanotubes. Proc. Natl. Acad. Sci. USA 2010, 107, 12369–12374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pardridge, W.M. The blood-brain barrier: Bottleneck in brain drug development. NeuroRx J. Am. Soc. Exp. Neurother. 2005, 2, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Grabrucker, A.M.; Ruozi, B.; Belletti, D.; Pederzoli, F.; Forni, F.; Vandelli, M.A.; Tosi, G. Nanoparticle transport across the blood brain barrier. Tissue Barriers 2016, 4, e1153568. [Google Scholar] [CrossRef] [PubMed]
- Lajoie, J.M.; Shusta, E.V. Targeting receptor-mediated transport for delivery of biologics across the blood-brain barrier. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 613–631. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, J. Mechanism of polymeric nanoparticle-based drug transport across the blood-brain barrier (BBB). J. Microencapsul. 2013, 30, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Zensi, A.; Begley, D.; Pontikis, C.; Legros, C.; Mihoreanu, L.; Buchel, C.; Kreuter, J. Human serum albumin nanoparticles modified with apolipoprotein A-I cross the blood-brain barrier and enter the rodent brain. J. Drug Target. 2010, 18, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Li, J.; Shen, Y.; Lu, W.; Gao, X.; Zhang, Q.; Jiang, X. Lactoferrin-conjugated PEG–PLA nanoparticles with improved brain delivery: In vitro and in vivo evaluations. J. Control. Release 2009, 134, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Ulbrich, K.; Hekmatara, T.; Herbert, E.; Kreuter, J. Transferrin-and transferrin-receptor-antibody-modified nanoparticles enable drug delivery across the blood–brain barrier (BBB). Eur. J. Pharm. Biopharm. 2009, 71, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Gil, S.; Andrieux, K.; Nicolas, V.; Appel, M.; Chacun, H.; Desmaële, D.; Taran, F.; Georgin, D.; Couvreur, P. Low-density lipoprotein receptor-mediated endocytosis of PEGylated nanoparticles in rat brain endothelial cells. Cell. Mol. Life Sci. 2007, 64, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Shilo, M.; Sharon, A.; Baranes, K.; Motiei, M.; Lellouche, J.-P.M.; Popovtzer, R. The effect of nanoparticle size on the probability to cross the blood–brain barrier: An in vitro endothelial cell model. J. Nanobiotechnol. 2015, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Lockman, P.R.; Koziara, J.M.; Mumper, R.J.; Allen, D.D. Nanoparticle surface charges alter blood–brain barrier integrity and permeability. J. Drug Target. 2004, 12, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Nagy, J.A.; Hipp, J.; Dvorak, H.F.; Dvorak, A.M. Vesiculo-vacuolar organelles and the regulation of venule permeability to macromolecules by vascular permeability factor, histamine, and serotonin. J. Exp. Med. 1996, 183, 1981–1986. [Google Scholar] [CrossRef]
- Golombek, S.K.; May, J.N.; Theek, B.; Appold, L.; Drude, N.; Kiessling, F.; Lammers, T. Tumor targeting via EPR: Strategies to enhance patient responses. Adv. Drug Deliv. Rev. 2018, 130, 17–38. [Google Scholar] [CrossRef]
- Basha, M. Nanotechnology as a promising strategy for anticancer drug delivery. Curr. Drug Deliv. 2018, 15, 497–509. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Rosenblum, D.; Joshi, N.; Tao, W.; Karp, J.M.; Peer, D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 2018, 9, 1410. [Google Scholar] [CrossRef]
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef]
- Sun, T.; Wu, H.; Li, Y.; Huang, Y.; Yao, L.; Chen, X.; Du, Z. Targeting transferrin receptor delivery of temozolomide for a potential glioma stem cell-mediated therapy. Oncotarget 2017, 8, 74451–74465. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.; Torchilin, V.P. Monoclonal antibody 2C5-modified doxorubicin-loaded liposomes with significantly enhanced therapeutic activity against intracranial human brain U-87 MG tumor xenografts in nude mice. Cancer Immunol. Immunother. 2007, 56, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Quadir, M.A.; Morton, S.W.; Mensah, L.B.; Shopsowitz, K.; Dobbelaar, J.; Effenberger, N.; Hammond, P.T. Ligand-decorated click polypeptide derived nanoparticles for targeted drug delivery applications. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1797–1808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morozova, O.V.; Pavlova, E.R.; Bagrov, D.V.; Barinov, N.A.; Prusakov, K.A.; Isaeva, E.I.; Podgorsky, V.V.; Basmanov, D.V.; Klinov, D.V. Protein nanoparticles with ligand-binding and enzymatic activities. Int. J. Nanomed. 2018, 13, 6637–6646. [Google Scholar] [CrossRef] [PubMed]
- Sayers, E.J.; Magnusson, J.P.; Moody, P.R.; Mastrotto, F.; Conte, C.; Brazzale, C.; Borri, P.; Caliceti, P.; Watson, P.; Mantovani, G.; et al. Switching of macromolecular ligand display by thermoresponsive polymers mediates endocytosis of multiconjugate nanoparticles. Bioconjug. Chem. 2018, 29, 1030–1046. [Google Scholar] [CrossRef] [PubMed]
- Alavizadeh, S.H.; Soltani, F.; Ramezani, M. Recent advances in immunoliposome-based cancer therapy. Curr. Pharmacol. Rep. 2016, 2, 129–141. [Google Scholar] [CrossRef]
- Rana, S.; Gallo, A.; Srivastava, R.S.; Misra, R.D.K. On the suitability of nanocrystalline ferrites as a magnetic carrier for drug delivery: Functionalization, conjugation and drug release kinetics. Acta Biomater. 2007, 3, 233–242. [Google Scholar] [CrossRef]
- Panja, S.; Maji, S.; Maiti, T.K.; Chattopadhyay, S. A smart magnetically active nanovehicle for on-demand targeted drug delivery: Where van der waals force balances the magnetic interaction. ACS Appl. Mater. Interfaces 2015, 7, 24229–24241. [Google Scholar] [CrossRef]
- Mansouri, M.; Nazarpak, M.H.; Solouk, A.; Akbari, S.; Hasani-Sadrabadi, M.M. Magnetic responsive of paclitaxel delivery system based on SPION and palmitoyl chitosan. J. Magn. Magn. Mater. 2017, 421, 316–325. [Google Scholar] [CrossRef] [Green Version]
- Ge, J.; Neofytou, E.; Cahill, T.J.; Beygui, R.E.; Zare, R.N. Drug release from electric-field-responsive nanoparticles. ACS Nano 2012, 6, 227–233. [Google Scholar] [CrossRef]
- Cantu, T.; Walsh, K.; Pattani, V.P.; Moy, A.J.; Tunnell, J.W.; Irvin, J.A.; Betancourt, T. Conductive polymer-based nanoparticles for laser-mediated photothermal ablation of cancer: Synthesis, characterization, and in vitro evaluation. Int. J. Nanomed. 2017, 12, 615–632. [Google Scholar] [CrossRef] [PubMed]
- Chandran, P.R.; Sandhyarani, N. An electric field responsive drug delivery system based on chitosan–gold nanocomposites for site specific and controlled delivery of 5-fluorouracil. RSC Adv. 2014, 4, 44922–44929. [Google Scholar] [CrossRef]
- Li, T.J.; Huang, C.C.; Ruan, P.W.; Chuang, K.Y.; Huang, K.J.; Shieh, D.B.; Yeh, C.S. In vivo anti-cancer efficacy of magnetite nanocrystal-based system using locoregional hyperthermia combined with 5-fluorouracil chemotherapy. Biomaterials 2013, 34, 7873–7883. [Google Scholar] [CrossRef] [PubMed]
- Thermodox. Available online: http://investor.celsion.com/news-releases/news-release-details/celsion-provides-update-thermodoxr-phase-iii-optima-study (accessed on 15 June 2019).
- Yin, W.; Yan, L.; Yu, J.; Tian, G.; Zhou, L.; Zheng, X.; Zhang, X.; Yong, Y.; Li, J.; Gu, Z.; et al. High-throughput synthesis of single-layer MoS2 nanosheets as a near-infrared photothermal-triggered drug delivery for effective cancer therapy. ACS Nano 2014, 8, 6922–6933. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.H.; Nguyen, H.T.; Pham, T.T.; Choi, J.Y.; Choi, H.G.; Yong, C.S.; Kim, J.O. Development of a graphene oxide nanocarrier for dual-drug chemo-phototherapy to overcome drug resistance in cancer. ACS Appl. Mater. Interfaces 2015, 7, 28647–28655. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Hao, J.; Lee, L.A.; Biewer, M.C.; Wang, Q.; Stefan, M.C. Thermally controlled release of anticancer drug from selfassembled γ-substituted amphiphilic poly(ε-caprolactone) micellar nanoparticles. Biomacromolecules 2012, 13, 2163–2173. [Google Scholar] [CrossRef] [PubMed]
- Vines, J.B.; Yoon, J.H.; Ryu, N.E.; Lim, D.J.; Park, H. Gold nanoparticles for photothermal cancer therapy. Front. Chem. 2019, 7, 167. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.H.; Kanasaki, K.; Gocheva, V.; Blum, G.; Harper, J.; Moses, M.A.; Shih, S.C.; Nagy, J.A.; Joyce, J.; Bogyo, M.; et al. VEGF-A induces angiogenesis by perturbing the cathepsin–cysteine protease inhibitor balance in venules, causing basement membrane degradation and mother vessel formation. Cancer Res. 2009, 69, 4537–4544. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Zhu, Y.; Gao, C.; Ling, C.; Qin, J.; Wang, Q.; Huang, Y.; Lu, W.; Wang, J. Menthol-modified BSA nanoparticles for glioma targeting therapy using an energy restriction strategy. NPG Asia Mater. 2019, 11, 38. [Google Scholar] [CrossRef]
- Sahel, N.A.; Habib-ur-Rehman, A. Enhancing cancer cell adhesion with clay nanoparticles for countering metastasis. Sci. Rep. 2019, 9, 5935. [Google Scholar] [CrossRef]
- Riley, R.S.; June, C.H.; Langer, R.; Mitchell, M.J. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 2019, 18, 175–196. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.P.; Biswas, A.; Shukla, A.; Maiti, P. Targeted therapy in chronic diseases using nanomaterial-based drug delivery vehicles. Signal Transduct. Target. Ther. 2019, 4, 33. [Google Scholar] [CrossRef]
- FDA Guidance. Drug Products, Including Biological Products, that Contain Nanomaterials—Guidance for Industry. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/drug-products-including-biological-products-contain-nanomaterials-guidance-industry (accessed on 30 July 2019).
- Choi, Y.H.; Han, H.-K. Nanomedicines: Current status and future perspectives in aspect of drug delivery and pharmacokinetics. J. Pharm. Investig. 2018, 48, 43–60. [Google Scholar] [CrossRef]
- Bor, G.; Mat Azmi, I.D.; Yaghmur, A. Nanomedicines for cancer therapy: Current status, challenges and future prospects. Ther. Deliv. 2019, 10, 113–132. [Google Scholar] [CrossRef] [PubMed]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-based medicines: A review of FDA-approved materials and clinical trials to date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Novel Drug Approvals for 2017. Available online: www.fda.gov/drugs/developmentapprovalprocess/druginnovation/ucm537040 (accessed on 20 July 2019).
- Kam, N.W.S.; Liu, Z.; Dai, H. Functionalization of carbon nanotubes via cleavable disulfide bonds for efficient intracellular delivery of siRNA and potent gene silencing. J. Am. Chem. Soc. 2005, 127, 12492–12493. [Google Scholar] [CrossRef]
- McCarthy, J.R.; Weissleder, R. Multifunctional magnetic nanoparticles for targeted imaging and therapy. Adv. Drug Deliv. Rev. 2008, 60, 1241–1251. [Google Scholar] [CrossRef] [Green Version]
- Symens, N.; Walczak, R.; Demeester, J.; Mattaj, I.; de Smedt, S.C.; Remaut, K. Nuclear inclusion of nontargeted and chromatin-targeted polystyrene beads and plasmid DNA containing nanoparticles. Mol. Pharm. 2011, 8, 1757–1766. [Google Scholar] [CrossRef]
- Hafner, A.; Lovrić, J.; Lakoš, G.P.; Pepić, I. Nanotherapeutics in the EU: An overview on current state and future directions. Int. J. Nanomed. 2014, 9, 1005–1023. [Google Scholar] [CrossRef]
- Nyström, A.M.; Fadeel, B. Safety assessment of nanomaterials: Implications for nanomedicine. J. Control. Release 2012, 161, 403–408. [Google Scholar] [CrossRef]







| Year of Approval | Type of Nanocarrier | Commercial Name/Producer | Indications | |
|---|---|---|---|---|
| Synthetic Polymer Nanoparticles | ||||
| 1990 | PEGylated adenosine deaminase enzyme | Adagen®/ pegademase bovine -Sigma-Tau Pharmaceuticals | Severe combined immunodeficiency disease (SCID) due to adenosine deaminase (ADA) deficiency | |
| 1994 | Polymer-protein conjugate PEGylated l-asparaginase | Oncaspar®/INN-pegaspargase-EnzonPharmaceuticals | Acute lymphoblastic leukaemia | |
| 1996 | Random copolymer of l-glutamate, l-alanine, l-lysine and l-tyrosine | Copaxone®/ Glatopa/glatiramer–Teva | Relapsing forms of multiple sclerosis | |
| 2000 | Poly(allylamine hydrochloride) | Renagel® [sevelamer HCl]/ [sevelamer carbonate] -Sanofi | Chronic kidney disease | |
| 2001 | PEGylated IFN alpha-2b protein | PegIntron®/INN-peginterferon alpha-2b-Merck | Chronic Hepatitis C | |
| 2002 | Leuprolide acetate and polymer [PLGH(poly(dl-lactide-coglycolide)] | Eligard®-Tolmar | Advanced prostate cancer | |
| 2002 | PEGylated IFN alpha-2a protein | Pegasys®-Genentech | Hepatitis B and C | |
| 2002 | PEGylated GCSF protein | Neulasta®/pegfilgrastim-Amgen | Prevention of Chemotherapy-induced Neutropenia | |
| 2003 | PEGylated HGH receptor antagonist | Somavert®/pegvisomant–Pfizer | Acromegaly | |
| 2004 | PEGylated anti-VEGF aptamer (vascular endothelial growth factor) aptamer | Macugen®/Pegaptanib-Bausch&Lomb | Neovascular (wet) age-related macular degeneration (AMD) in adults | |
| 2007 | Chemically synthesized ESA (erythropoiesis-stimulating agent) | Mircera®/Methoxy PEG glycol-epoetin β-Hoffman-LaRoche | Anemia associated with chronic kidney disease | |
| 2008 2009 2013 | PEGylated antibody fragment (Certolizumab) | Cimzia®/ Certolizumabpegol-UCB | Chron’s disease, rheumatoid arthritis, active psoriatic arthritis, active ankylosing spondylitis | |
| 2010 | Polymer-protein conjugate (PEGylated porcine-likeuricase) | Krystexxa®/pegloticase–Horizon | Chronic gout | |
| 2014 | Polymer-protein conjugate (PEGylated IFNbeta-1a) | Plegridy®-Biogen | Relapsing remitting multiple sclerosis | |
| 2015 | Polymer-protein conjugate (PEGylated factor VIII) | Adynovate-Baxalta | Hemophilia A | |
| Liposomes | ||||
| 1995 2005 2008 | Liposomal doxorubicin | Doxil®/Caelyx™-Janssen | Karposi sarcoma, ovarian cancer, Multiple myeloma | |
| 1995 | Liposomal amphotericinB lipid complex | Abelcet®-Sigma-Tau | Invasive fungal infections in patients who are refractory to or intolerant of conventional amphotericin B | |
| 1996 | Liposomal daunorubicin | DaunoXome®-Galen | Karposi sarcoma | |
| 1996 | Liposomal cytarabine | DepoCyt©-Sigma-Tau | Lymphoma | |
| 1997 | Liposomal amphotericin B | AmBisome®-Gilead Sciences | Fungal infection in febrile, neutropenic patients | |
| 1999 | Liposome-proteins SP-band SP-C | Curosurf®/Poractantalpha -Chieseifarmaceutici | Rescue treatment of Respiratory Distress Syndrome (RDS) in premature infants | |
| 2000 | Liposomal verteporfin | Visudyne®-Bauschand Lomb | Subfoveal choroidal neovascularization due to age-related macular degeneration | |
| 2004 | Liposomal morphine sulphate | DepoDur®-Pacira Pharmaceuticals | Treatment of pain following major surgery | |
| 2012 | Liposomal vincristine | Marqibo®-Onco TCS | Treatment of adult patients with Philadelphia chromosome-negative (Ph-) acute lymphoblastic leukemia (ALL) in second or greater relapse or whose disease has progressed following two or more anti-leukemia therapies | |
| 2015 | Liposomal irinotecan | Onivyde® Merrimack | Pancreatic cancer | |
| Micelles | ||||
| 2003 | Micellar estradiol | Estrasorb™-Novavax | Moderate to severe vasomotor symptoms associated with menopause | |
| Protein NPs | ||||
| 1999 | Engineered protein combining L-2 and diphtheria toxin | Ontak®-Eisai Inc | T-Cell lymphoma | |
| 2005 2012 2013 | Albumin-bound paclitaxel NP | Abraxane®/ABI-007–Celgene | Breast cancer, non-small cell lung cancer, pancreatic cancer | |
| Nanocrystals | ||||
| 2000 | Sirolimus | Rapamune®-Wyeth Pharmaceuticals | Immunosupressant | |
| 2001 | Megestrol acetate | MegaceES®-Par Pharmaceuticals | Anorexia | |
| 2002 | Metyhlphenidate HCl | Ritalin LA®-Novartis | Attention Deficit Hyperactivity Disorder | |
| 2002 | Tizanidine HCl | Zanaflex®-Acorda | Muscle relaxant | |
| 2002 2015 | Morphine sulfate | Avinza®-Pfizer | Narcotic pain reliever | |
| 2003 | Calcium phosphate | Vitoss®-Stryker | Bone graft substitute | |
| 2003 | Aprepitant | Emend®-Merck | Antiemetic agent | |
| 2003 | Hydroxyapatite | OsSatura® IsoTis Orthobiologics | Bone substitute | |
| 2004 | Fenofibrate | Tricor®-Lupin Atlantis | Hyperlipidemia | |
| 2004 | Hydroxyapatite | Ostim®-Heraseus Kulzer | Bone substitute | |
| 2005 | Dexamethyl-phenidate HCl | Focalin XR®-Novartis | Attention Deficit Hyperactivity Disorder (ADHD) as mental stimulant | |
| 2005 | Hydroxyapatite | NanOss®-Rti Surgical | Bone substitute | |
| 2009 | Hydroxyapatite | EquivaBone® Zimme-Biomet | Bone substitute | |
| 2009 2014 | Paliperidone Palmitate | Invega®Sustenna®-Janssen Pharms | Schizoaffective disorder | |
| 2014 | Dantrolene sodium | Ryanodex®-Eagle Pharmaceuticals | Malignant hyperthermia | |
| Inorganic/Metallic NPs | ||||
| 1995 | Iron dextran (low MW) | INFeD®-Sanofi Avertis | Iron-deficiency anemia | |
| 1996 | SPION coated with dextran | Feridex®/Endorem®-AMAG pharmaceuticals | Imaging materials | |
| 1997 | Iron dextran (high MW) | DexIron®/Dexferrum®-Sanofi Avertis | Iron deficiency anemia, hemodialysis-induced | |
| 1999 | Sodium ferric gluconate | Ferrlecit®-Sanofi Avertis | Iron deficiency anemia in adult patients and in pediatric patients age 6 years and older with chronic kidney disease receiving hemodialysis who are receiving supplemental epoetin therapy | |
| 2000 | Iron sucrose | Venofer®-Luitpold Pharmaceuticals | Iron deficiency anemia in patients with chronic kidney disease | |
| 2008 | SPION coated with dextran | GastroMARK™/umirem®-AMAG pharmaceuticals | Imaging materials | |
| 2009 | Ferumoxytol SPION with poly glucose sorbitol carboxy methylether | Feraheme™/ferumoxytol-AMAG pharmaceuticals | Iron deficiency anemia in patients with chronic kidney disease | |
| 2010 | Iron oxide | Nanotherm®-MagForce | Hybrid species | |
| Year of Approval | Type of Nanocarrier | Active Principle- Commercial Name | Indications |
|---|---|---|---|
| 1994 (Japan) | Polymer protein conjugate | Styrene maleic anhydride neocarzinostatin (SMANCS)- Zinostatin stimalamer | Renal and hepatic cancer |
| 1996 | Iron oxide NPs | Ferumoxides–Feridex | i.v. administration as an adjunct to MRI (in adult patients) to enhance the T2 weighted images used in the detection and evaluation of lesions of the liver that are associated with an alteration in the RES |
| 1995 (FDA) 1996 (EMA) | Liposome (PEGylated) | Doxorubicin-Doxil/Caelyx | HIV-associated Kaposi’s sarcoma, ovarian cancer, metastatic breast cancer, multiple myeloma |
| 1996 (FDA) | Liposome (non- PEGylated) | Daunorubicin-DaunoXome | HIV-associated Kaposi’s Sarcoma |
| 1998 (Taiwan) | Liposome | Doxorubicin-Lipo-Dox | Kaposi’s sarcoma, breast and ovarian cancer |
| 1999 (FDA) | Liposome | Cytosine arabinoside (cytarabine)–DepoCyt | Neoplastic meningitis |
| 2000 (EMA) | Liposome | Doxorubicin-Myocet | Breast cancer |
| 2005 (FDA) 2008 (EMA) | Nanoparticle albumin Bound | Paclitaxel-Abraxane | Advanced non-small-cell lung cancer, metastatic pancreatic cancer, metastatic breast cancer |
| 2006 (FDA) | PEG protein conjugate | l-Asparaginase–Oncaspar | Leukemia |
| 2007 (South Korea) | PEG-PLA polymeric Micelle | Paclitaxel-Genexol-PM | Breast cancer, Lung cancer, Ovarian cancer |
| 2002 | Polymeric NP | Leuprolide acetate–Eligard | Prostate cancer |
| 2009 (EMA) | Liposome (non- PEGylated) | Mifamurtide–MEPACT | Osteosarcoma |
| 2010 (EMA) | Iron oxide nanoparticle | NanoTherm | Glioblastoma |
| 2012 (FDA) | Liposome (non- PEGylated) | Vincristine-Marqibo | Philadelphia chromosome negative acute lymphoblastic leukemia |
| 2015 (FDA) | Liposome (PEGylated) | Irinotecan/MM-398–Onivyde | Metastatic pancreatic cancer |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sevastre, A.-S.; Horescu, C.; Carina Baloi, S.; Cioc, C.E.; Vatu, B.I.; Tuta, C.; Artene, S.A.; Danciulescu, M.M.; Tudorache, S.; Dricu, A. Benefits of Nanomedicine for Therapeutic Intervention in Malignant Diseases. Coatings 2019, 9, 628. https://doi.org/10.3390/coatings9100628
Sevastre A-S, Horescu C, Carina Baloi S, Cioc CE, Vatu BI, Tuta C, Artene SA, Danciulescu MM, Tudorache S, Dricu A. Benefits of Nanomedicine for Therapeutic Intervention in Malignant Diseases. Coatings. 2019; 9(10):628. https://doi.org/10.3390/coatings9100628
Chicago/Turabian StyleSevastre, Ani-Simona, Cristina Horescu, Stefania Carina Baloi, Catalina Elena Cioc, Bogdan Ionel Vatu, Cristian Tuta, Stefan Alexandru Artene, Maria Mihaela Danciulescu, Stefania Tudorache, and Anica Dricu. 2019. "Benefits of Nanomedicine for Therapeutic Intervention in Malignant Diseases" Coatings 9, no. 10: 628. https://doi.org/10.3390/coatings9100628
APA StyleSevastre, A. -S., Horescu, C., Carina Baloi, S., Cioc, C. E., Vatu, B. I., Tuta, C., Artene, S. A., Danciulescu, M. M., Tudorache, S., & Dricu, A. (2019). Benefits of Nanomedicine for Therapeutic Intervention in Malignant Diseases. Coatings, 9(10), 628. https://doi.org/10.3390/coatings9100628