Investigation of High Voltage Anodic Plasma (HVAP) Ag-DLC Coatings on Ti50Zr with Different Ag Amounts
Abstract
:1. Introduction
2. Materials and Methods
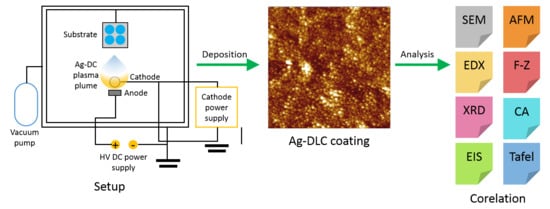
2.1. Thin Film Deposition
2.2. Surface Analysis
2.3. Electrochemical Stability
3. Results and Discussion
3.1. Surface Characterization
3.2. Film Composition
3.3. Electrochemical Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Darvell, B.W. Materials Science for Dentistry, 9th ed.; Woodhead Publishing: Cambridge, UK, 2009. [Google Scholar]
- Ionita, D.; Grecu, M.; Ungureanu, C.; Demetrescu, I. Modifying the TiAlZr biomaterial surface with coating, for a better anticorrosive and antibacterial performance. Appl. Surf. Sci. 2011, 257, 9164–9168. [Google Scholar] [CrossRef]
- Bernhard, N.; Berner, S.; De Wild, M.; Wieland, M. The binary (TiZr) alloy—A newly developed (Ti) alloy for use in dental implant. Forum Implant. 2009, 5, 30–39. [Google Scholar]
- Liu, X.; Chen, S.; Tsoi, J.K.H.; Matinlinna, J.P. Binary titanium alloys as dental implant materials—A review. Regen. Biomater. 2017, 4, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Gradin, H.M.; Berner, S.; Dard, M. A review of titanium zirconium (TiZr) alloys for use in endosseous dental implants. Materials 2012, 5, 1348–1360. [Google Scholar] [CrossRef]
- Ionita, D.; Golgovici, F.; Mazare, A.; Badulescu, M.; Demetrescu, I.; Pandelea-Dobrovicescu, G.-R. Corrosion and antibacterial characterization of Ag-DLC coatingon a new CoCrNbMoZr dental alloy. Mater. Corros 2018, 69, 1403–1411. [Google Scholar] [CrossRef]
- Mazare, A.; Anghel, A.; Surdu-Bob, C.; Totea, G.; Demetrescu, I.; Ionita, D. Silver doped diamond-like carbon antibacterial and corrosion resistance coatings on titanium. Thin Solid Film. 2018, 657, 16–23. [Google Scholar] [CrossRef]
- Izquierdo, J.; Bolat, G.; Mareci, D.; Munteanu, C.; González, S.; Souto, R. Electrochemical behaviour of ZrTi alloys in artificial physiological solution simulating in vitro inflammatory conditions. Appl. Surf. Sci. 2014, 313, 259–266. [Google Scholar] [CrossRef]
- Akimoto, T.; Ueno, T.; Tsutsumi, Y.; Doi, H.; Hanawa, T.; Wakabayashi, N. Evaluation of corrosion resistance of implant-use Ti–Zr binary alloys with a range of compositions. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 73–79. [Google Scholar] [CrossRef]
- Zhou, Y.K.; Jing, R.; Ma, M.Z.; Liu, R.P. Tensile strength of (Zr-Ti) binary alloy. Chin. Phys. Lett. 2013, 30, 116201. [Google Scholar] [CrossRef]
- Cui, W.; Liu, Y. Fatigue behavior of Ti50Zr alloy for dental implant application. J. Alloys Compd. 2019, 793, 212–219. [Google Scholar] [CrossRef]
- Romonti, D.E.; Gomez Sanchez, A.V.; Milošev, I.; Demetrescu, I.; Ceré, S. Effect of anodization on the surface characteristics and electrochemical behaviour of Zr in artificial saliva. Mater. Sci. Eng. C 2016, 62, 458–466. [Google Scholar] [CrossRef]
- Sivaraman, K.; Chopra, A.; Narayan, A.I.; Balakrishnan, D. Is zirconia a viable alternative to titanium for oral implant? A critical review. J. Prosthodont. Res. 2018, 62, 121–133. [Google Scholar] [CrossRef]
- Schünemann, F.H.; Galárraga-Vinueza, M.E.; Magini, R.; Fredel, M.; Silva, F.; Souza, J.C.M.; Zhang, Y.; Henriques, B. Zirconia surface modifications for implant dentistry. Mater. Sci. Eng. C 2019, 98, 1294–1305. [Google Scholar] [CrossRef] [PubMed]
- Ionita, D.; Vardaki, M.; Stan, M.S.; Dinischiotu, A.; Demetrescu, I. Enhance stability and in vitro cell response to a bioinspired coating on zr alloy with increasing chitosan content. J. Bionic Eng. 2017, 14, 459–467. [Google Scholar] [CrossRef]
- Busuioc, C.; Voicu, G.; Zuzu, I.D.; Miu, D.; Sima, S.; Iordache, F.; Jinga, S.I. Vitroceramic coatings deposited by laser ablation on Ti–Zr substrates for implantable medical applications with improved biocompatibility. Ceram. Int. 2017, 43, 5498–5504. [Google Scholar] [CrossRef]
- Písařík, P.; Jelínek, M.; Remsa, J.; Mikšovský, J.; Zemek, J.; Jurek, K.; Kubinová, Š.; Lukeš, J.; Šepitka, J. Antibacterial, mechanical and surface properties of Ag-DLC films prepared by dual PLD for medical applications. Mater. Sci. Eng. C 2017, 77, 955–962. [Google Scholar] [CrossRef]
- Hatada, R.; Flege, S.; Rimmler, B.; Dietz, C.; Ensinger, W.; Baba, K. Surface structuring of diamond-like carbon films by chemical etching of zinc inclusions. Coatings 2019, 9, 125. [Google Scholar] [CrossRef]
- Manninen, N.K.; Ribeiro, F.; Escudeiro, A.; Polcar, T.; Carvalho, S.; Cavaleiro, A. Influence of Ag content on mechanical and tribological behavior of DLC coatings. Surf. Coat. Technol. 2013, 232, 440–446. [Google Scholar] [CrossRef]
- Wang, L.J.; Zhang, F.; Fong, A.; Lai, K.M.; Shum, P.W.; Zhou, Z.F.; Gao, Z.F.; Fu, T. Effects of silver segregation on sputter deposited antibacterial silver-containing diamond-like carbon films. Thin Solid Film. 2018, 650, 58–64. [Google Scholar] [CrossRef]
- Cloutier, M.; Turgeon, S.; Busby, Y.; Tatoulian, M.; Pireaux, J.J.; Mantovani, D. Controlled distribution and clustering of silver in Ag-DLC nanocomposite coatings using a hybrid plasma approach. ACS Appl. Mater. Interfaces 2016, 8, 21020–21027. [Google Scholar] [CrossRef]
- Cloutier, M.; RTolouei, R.; OLesage, O.; Lévesque, L.; Turgeon, S.; Tatoulian, M.; Mantovani, D. On the long term antibacterial features of silver-doped diamond like carbon coatings deposited via a hybrid plasma process. Biointerphases 2014, 9, 029013. [Google Scholar] [CrossRef] [PubMed]
- Surdu-Bob, C.; Mustata, I.; Iacob, C. General characteristics of the Thermoionic Vacuum Arc plasma. J. Optoelectron. Adv. Mater. 2007, 9, 2932–2934. [Google Scholar]
- Surdu-Bob, C.; Vladoiu, R.; Badulescu, M.; Musa, G. Control over the sp2/sp3 ratio by tuning plasma parameters of the thermoionic vacuum arc. Diam. Relat. Mater. 2008, 17, 1625–1628. [Google Scholar] [CrossRef]
- Choi, H.W.; Choi, J.H.; Lee, K.R.; Ahn, J.P.; Oh, K.H. Structure and mechanical properties of Ag-incorporated DLC films prepared by a hybrid ion beam deposition system. Thin Solid Film. 2007, 516, 248–251. [Google Scholar] [CrossRef]
- Manninen, N.; Escobar Galindo, R.; Carvalho, S.; Cavaleiro, A. Silver surface segregation in Ag-DLC nanocomposite coatings. Surf. Coat. Technol. 2014, 267, 90–97. [Google Scholar] [CrossRef]
- Chakravadhanula, V.S.; Kübel, C.; Hrkac, T.; Zaporojtchenko, V.; Strunskus, T.; Faupel, F.; Kienle, L. Surface segregation in TiO2-based nanocomposite thin films. Nanotechnology 2012, 23, 495701. [Google Scholar] [CrossRef]
- Li, D.; Lin, C.; Batchelor-McAuley, C.; Chen, L.; Compton, R.G. Tafel analysis in practice. J. Electroanal. Chem. 2018, 826, 117–124. [Google Scholar] [CrossRef]
- Popa, M.V.; Demetrescu, I.; Vasilescu EIonita, D.; Vasilescu, C. Stability of some dental materials in oral biofluids. Rev. Roum. Chim. 2005, 50, 399–406. [Google Scholar]
- Milošev, I.; Kosec, T.; Strehblow, H.H. XPS and EIS study of passive film formed on orthopaedic Ti–6Al–7Nb alloy in Hank’s physiological solution. Electrochim. Acta 2008, 53, b3547–b3558. [Google Scholar] [CrossRef]
- Demetrescu, I.; Pirvu, C.; Mitran, V. Effect of nano-topographical features of Ti/TiO2 electrode surface on cell response and electrochemical stability in artificial saliva. Bioelectrochemistry 2010, 79, 122–129. [Google Scholar] [CrossRef]
- Ponsonnet, L.; Reybier, K.; Jaffrezic, N.; Comte, V.; Lagneau, C.; Lissac, M.; Martelet, C. Relationship between surface properties (roughness, wettability) of titanium and titanium alloys and cell behavior. Mater. Sci. Eng. C 2003, 23, 551–560. [Google Scholar] [CrossRef]








| Sample | A1 | A2 | A3 | A4 |
|---|---|---|---|---|
| Ag in anode (%) | 0 | 1.8 | 5.6 | 8.3 |
| Deposition rate nm·s−1 | 1.33 | 2.4 | 0.15 | 0.13 |
| Sample | Rsol [Ω·cm2] | Rpor [KΩ·cm2] | Ccoat [µF·cm2] | n1 | R1 [KΩ·cm2] | Cdl [µF·cm2] | n2 |
|---|---|---|---|---|---|---|---|
| A1 | 266 | 106.2 | 6.48 | 0.792 | 277.0 | 22.62 | 0.861 |
| A2 | 262 | 204.9 | 3.86 | 0.854 | 616.51 | 18.6 | 0.823 |
| A3 | 252 | 305.6 | 2.01 | 0.898 | 968.25 | 10.24 | 0.712 |
| A4 | 258 | 386.8 | 1.62 | 0.878 | 1098.12 | 8.32 | 0.789 |
| Sample | Icorr (nA·cm−2) | Ecorr (V) | Vcorr (μm∙year−1) | Rp (MOhm·cm) |
|---|---|---|---|---|
| A1 | 47.11 | −67.40 | 4.94 | 1.813 |
| A2 | 37.02 | −274.47 | 4.73 | 3.32 |
| A3 | 6.26 | −157.39 | 1.89 | 5.233 |
| A4 | 4.26 | −193.43 | 1.87 | 5.614 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoian, A.B.; Surdu-Bob, C.; Anghel, A.; Ionita, D.; Demetrescu, I. Investigation of High Voltage Anodic Plasma (HVAP) Ag-DLC Coatings on Ti50Zr with Different Ag Amounts. Coatings 2019, 9, 792. https://doi.org/10.3390/coatings9120792
Stoian AB, Surdu-Bob C, Anghel A, Ionita D, Demetrescu I. Investigation of High Voltage Anodic Plasma (HVAP) Ag-DLC Coatings on Ti50Zr with Different Ag Amounts. Coatings. 2019; 9(12):792. https://doi.org/10.3390/coatings9120792
Chicago/Turabian StyleStoian, Andrei Bogdan, Cristina Surdu-Bob, Alexandru Anghel, Daniela Ionita, and Ioana Demetrescu. 2019. "Investigation of High Voltage Anodic Plasma (HVAP) Ag-DLC Coatings on Ti50Zr with Different Ag Amounts" Coatings 9, no. 12: 792. https://doi.org/10.3390/coatings9120792
APA StyleStoian, A. B., Surdu-Bob, C., Anghel, A., Ionita, D., & Demetrescu, I. (2019). Investigation of High Voltage Anodic Plasma (HVAP) Ag-DLC Coatings on Ti50Zr with Different Ag Amounts. Coatings, 9(12), 792. https://doi.org/10.3390/coatings9120792