Silica Scaling Inhibition in Water Treatment Process Using Fibrous Al2O3-Nylon 6 Adsorbents
Abstract
:1. Introduction
2. Results and Discussion
2.1. Fabrication of Al2O3-Nylon 6 Composite Fibers
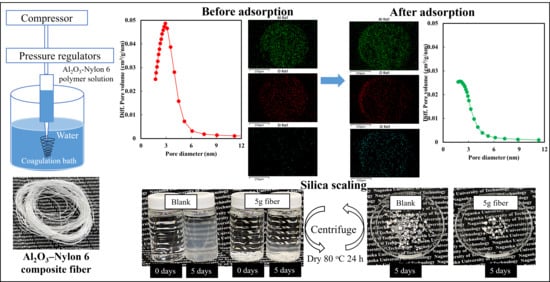
2.2. Silica Adsorption Behavior of Al2O3-Nylon 6 Composite Fibers
2.3. Silica Scaling Inhibition Effect of Al2O3-Nylon 6 Composite Fiber
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Characterizations of Al2O3-Nylon 6 Composite Fibers
4.3. Batch Adsorption Experiment of Silica
4.4. Scaling Inhibition Effect of Al2O3-Nylon 6 Composite Fibers
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gallup, D.L.; von Hirtz, P. Chapter 22—Control of Silica-Based Scales in Cooling and Geothermal Systems. In Mineral Scales and Deposits; Amjad, Z., Demadis, K.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 573–582. ISBN 978-0-444-63228-9. [Google Scholar]
- Hafez, O.M.; Shoeib, M.A.; El-Khateeb, M.A.; Abdel-Shafy, H.I.; Youssef, A.O. Removal of Scale Forming Species from Cooling Tower Blowdown Water by Electrocoagulation Using Different Electrodes. Chem. Eng. Res. Des. 2018, 136, 347–357. [Google Scholar] [CrossRef]
- Zarrouk, S.J.; Woodhurst, B.C.; Morris, C. Silica Scaling in Geothermal Heat Exchangers and Its Impact on Pressure Drop and Performance: Wairakei Binary Plant, New Zealand. Geothermics 2014, 51, 445–459. [Google Scholar] [CrossRef]
- Setiawan, F.A.; Rahayuningsih, E.; Petrus, H.T.B.M.; Nurpratama, M.I.; Perdana, I. Kinetics of Silica Precipitation in Geothermal Brine with Seeds Addition: Minimizing Silica Scaling in a Cold Re-Injection System. Geotherm. Energy 2019, 7, 22. [Google Scholar] [CrossRef]
- Braun, G.; Hater, W.; Zum Kolk, C.; Dupoiron, C.; Harrer, T.; Götz, T. Investigations of Silica Scaling on Reverse Osmosis Membranes. Desalination 2010, 250, 982–984. [Google Scholar] [CrossRef]
- Milne, N.A.; O’Reilly, T.; Sanciolo, P.; Ostarcevic, E.; Beighton, M.; Taylor, K.; Mullett, M.; Tarquin, A.J.; Gray, S.R. Chemistry of Silica Scale Mitigation for RO Desalination with Particular Reference to Remote Operations. Water Res. 2014, 65, 107–133. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.; Lee, Y.J.; Sheikholeslami, R. Silica Fouling and Cleaning of Reverse Osmosis Membranes. Desalination 2001, 139, 43–56. [Google Scholar] [CrossRef]
- Lunevich, L. Aqueous Silica and Silica Polymerisation. In Desalination; Farahani, M.H.D.A., Vatanpour, V., Taheri, A.H., Eds.; IntechOpen: Rijeka, Croatia, 2019; Chapter 6; ISBN 978-1-78984-739-0. [Google Scholar]
- Davis, S.N. Silica in Streams and Ground Water. Am. J. Sci. 1964, 262, 870–891. [Google Scholar] [CrossRef]
- Tong, T.; Zhao, S.; Boo, C.; Hashmi, S.M.; Elimelech, M. Relating Silica Scaling in Reverse Osmosis to Membrane Surface Properties. Environ. Sci. Technol. 2017, 51, 4396–4406. [Google Scholar] [CrossRef]
- Sahachaiyunta, P.; Koo, T.; Sheikholeslami, R. Effect of Several Inorganic Species on Silica Fouling in RO Membranes. Desalination 2002, 144, 373–378. [Google Scholar] [CrossRef]
- Park, Y.-M.; Yeon, K.-M.; Park, C. Silica Treatment Technologies in Reverse Osmosis for Industrial Desalination: A Review. Environ. Eng. Res. 2020, 25, 819–829. [Google Scholar] [CrossRef]
- Tong, T.; Wallace, A.F.; Zhao, S.; Wang, Z. Mineral Scaling in Membrane Desalination: Mechanisms, Mitigation Strategies, and Feasibility of Scaling-Resistant Membranes. J. Memb. Sci. 2019, 579, 52–69. [Google Scholar] [CrossRef]
- Lisitsin, D.; Hasson, D.; Semiat, R. Critical Flux Detection in a Silica Scaling RO System. Desalination 2005, 186, 311–318. [Google Scholar] [CrossRef]
- Shimelis, B.; Gungure, A.; Jule, L.; Bekele, B.; Redi, M.; Esakkiraj, E.; Balasubramaniam, S.; Ramaswamy, K.; Tesfaye, L. Preparation of Hydrated Lime Quality for Water Treatment: To Reduce Silica Concentration from Hydrated Lime up to Standard Specification. Desalination Water Treat. 2022, 251, 35–42. [Google Scholar] [CrossRef]
- Williams, M.; Evangelista, R.; Cohen, Y. Non-Thermal Process for Recovering Reverse Osmosis Concentrate: Process Chemistry and Kinetics. In Proceedings of the 2002 AWWA Water Quality Technology Conference, Seattle, WA, USA, 12–14 November 2002. [Google Scholar]
- Al-Rehaili, A.M. Comparative Chemical Clarification for Silica Removal from RO Groundwater Feed. Desalination 2003, 159, 21–31. [Google Scholar] [CrossRef]
- Bush, J.A.; Vanneste, J.; Gustafson, E.M.; Waechter, C.A.; Jassby, D.; Turchi, C.S.; Cath, T.Y. Prevention and Management of Silica Scaling in Membrane Distillation Using pH Adjustment. J. Memb. Sci. 2018, 554, 366–377. [Google Scholar] [CrossRef]
- Ben Sik Ali, M.; Hamrouni, B.; Bouguecha, S.; Dhahbi, M. Silica Removal Using Ion-Exchange Resins. Desalination 2004, 167, 273–279. [Google Scholar] [CrossRef]
- Salvador Cob, S.; Hofs, B.; Maffezzoni, C.; Adamus, J.; Siegers, W.G.; Cornelissen, E.R.; Genceli Güner, F.E.; Witkamp, G.J. Silica Removal to Prevent Silica Scaling in Reverse Osmosis Membranes. Desalination 2014, 344, 137–143. [Google Scholar] [CrossRef]
- Rahardianto, A.; Gao, J.; Gabelich, C.J.; Williams, M.D.; Cohen, Y. High Recovery Membrane Desalting of Low-Salinity Brackish Water: Integration of Accelerated Precipitation Softening with Membrane RO. J. Membr. Sci. 2007, 289, 123–137. [Google Scholar] [CrossRef]
- Cheng, H.-H.; Chen, S.-S.; Yang, S.-R. In-Line Coagulation/Ultrafiltration for Silica Removal from Brackish Water as RO Membrane Pretreatment. Sep. Purif. Technol. 2009, 70, 112–117. [Google Scholar] [CrossRef]
- Miranda, R.; Latour, I.; Blanco, A. Silica Removal from a Paper Mill Effluent by Adsorption on Pseudoboehmite and γ-Al2O3. Water 2021, 13, 2031. [Google Scholar] [CrossRef]
- Sasan, K.; Brady, P.V.; Krumhansl, J.L.; Nenoff, T.M. Exceptional Selectivity for Dissolved Silicas in Industrial Waters Using Mixed Oxides. J. Water Process Eng. 2017, 20, 187–192. [Google Scholar] [CrossRef]
- Bouguerra, W.; Ben Sik Ali, M.; Hamrouni, B.; Dhahbi, M. Equilibrium and Kinetic Studies of Adsorption of Silica onto Activated Alumina. Desalination 2007, 206, 141–146. [Google Scholar] [CrossRef]
- Sanciolo, P.; Milne, N.; Taylor, K.; Mullet, M.; Gray, S. Silica Scale Mitigation for High Recovery Reverse Osmosis of Groundwater for a Mining Process. Desalination 2014, 340, 49–58. [Google Scholar] [CrossRef]
- Nakamoto, K.; Ohshiro, M.; Kobayashi, T. Continuous Flow Column Adsorption of Mordenite Zeolite–Polymer Compositfibers for Lead Removal. Desalination Water Treat. 2018, 109, 297–306. [Google Scholar] [CrossRef]
- Kobayashi, T.; Ohshiro, M.; Nakamoto, K.; Uchida, S. Decontamination of Extra-Diluted Radioactive Cesium in Fukushima Water Using Zeolite–Polymer Composite Fibers. Ind. Eng. Chem. Res. 2016, 55, 6996–7002. [Google Scholar] [CrossRef]
- Nakamoto, K.; Ohshiro, M.; Kobayashi, T. Mordenite Zeolite—Polyethersulfone Composite Fibers Developed for Decontamination of Heavy Metal Ions. J. Environ. Chem. Eng. 2017, 5, 513–525. [Google Scholar] [CrossRef]
- Özdilek, C.; Kazimierczak, K.; van der Beek, D.; Picken, S.J. Preparation and Properties of Polyamide-6-Boehmite Nanocomposites. Polymer 2004, 45, 5207–5214. [Google Scholar] [CrossRef]
- Elmaaty, T.A.; El-Aziz, E.A.; Ma, J.; El-Taweel, F.; Okubayashi, S. Eco-Friendly Disperse Dyeing and Functional Finishing of Nylon 6 Using Supercritical Carbon Dioxide. Fibers 2015, 3, 309–322. [Google Scholar] [CrossRef]
- Illing, T.; Gotzig, H.; Schoßig, M.; Bierögel, C.; Grellmann, W. Influence of Hygrothermal Aging on Poisson’s Ratio of Thin Injection-Molded Short Glass Fiber-Reinforced PA6. Fibers 2016, 4, 17. [Google Scholar] [CrossRef]
- Rogalski, J.J.; Bastiaansen, C.W.M.; Peijs, T. PA6 Nanofibre Production: A Comparison between Rotary Jet Spinning and Electrospinning. Fibers 2018, 6, 37. [Google Scholar] [CrossRef]
- Dechojarassri, D.; Komatsu, K.; Sawara, A.; Tamura, H.; Furuike, T. Antimicrobial Properties of AgNP/TEMPO-Oxidized Cellulose Nanofiber/Chitosan Composite Fibers. Fibers 2023, 11, 69. [Google Scholar] [CrossRef]
- Yalcinkaya, F. Preparation of Various Nanofiber Layers Using Wire Electrospinning System. Arab. J. Chem. 2019, 12, 5162–5172. [Google Scholar] [CrossRef]
- Nakamoto, K.; Kobayashi, T. Fibrous Mordenite Zeolite—Polymer Composite Adsorbents to Methylene Blue Dye. Int. J. Eng. Tech. Res. 2017, 7, 264902. [Google Scholar]
- Le Thi, A.P.; Wakasugi, R.; Kobayashi, T. Suppression of Hydrogen Sulfide Generation via the Coexistence of Anaerobic Sludge and Goethite-Rich Limonite/Polyethersulfone Composite Fibers. ACS Omega 2023, 8, 35054–35065. [Google Scholar] [CrossRef] [PubMed]
- Ton Nu, P.T.; Kobayashi, T. Methanol/CaCl2 Wet Phase Inversion for Selective Separation of Heavy Metal Ions by Nylon 6-Mordenite Zeolite Composite Membranes. Int. J. Environ. Res. 2020, 14, 667–683. [Google Scholar] [CrossRef]
- Ton Nu, P.T.; Kobayashi, T. Nylon-6-Mordenite Composite Membranes for Adsorption of Ethylene Gas Released from Chiquita Bananas. Ind. Eng. Chem. Res. 2020, 59, 8212–8222. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Nakajima, L.; Yusof, N.N.M.; Kobayashi, T. Calixarene-Composited Host–Guest Membranes Applied for Heavy Metal Ion Adsorbents. Arab. J. Sci. Eng. 2015, 40, 2881–2888. [Google Scholar] [CrossRef]
- Joshi, M.K.; Tiwari, A.P.; Maharjan, B.; Won, K.S.; Kim, H.J.; Park, C.H.; Kim, C.S. Cellulose Reinforced Nylon-6 Nanofibrous Membrane: Fabrication Strategies, Physicochemical Characterizations, Wicking Properties and Biomimetic Mineralization. Carbohydr. Polym. 2016, 147, 104–113. [Google Scholar] [CrossRef]
- Zhang, H.; Li, S.; Branford White, C.J.; Ning, X.; Nie, H.; Zhu, L. Studies on Electrospun Nylon-6/Chitosan Complex Nanofiber Interactions. Electrochim. Acta 2009, 54, 5739–5745. [Google Scholar] [CrossRef]
- Liu, C.; Shih, K.; Gao, Y.; Li, F.; Wei, L. Dechlorinating Transformation of Propachlor through Nucleophilic Substitution by Dithionite on the Surface of Alumina. J. Soils Sediments 2012, 12, 724–733. [Google Scholar] [CrossRef]
- Swedlund, P.J.; Miskelly, G.M.; McQuillan, A.J. An Attenuated Total Reflectance IR Study of Silicic Acid Adsorbed onto a Ferric Oxyhydroxide Surface. Geochim. Cosmochim. Acta 2009, 73, 4199–4214. [Google Scholar] [CrossRef]
- Gaggiano, R.; De Graeve, I.; Mol, J.M.C.; Verbeken, K.; Kestens, L.A.I.; Terryn, H. An Infrared Spectroscopic Study of Sodium Silicate Adsorption on Porous Anodic Alumina. Surf. Interface Anal. 2013, 45, 1098–1104. [Google Scholar] [CrossRef]
- Chan, S.H. A Review on Solubility and Polymerization of Silica. Geothermics 1989, 18, 49–56. [Google Scholar] [CrossRef]









| Al2O3 Content (wt%) | Tensile Strength (MPa) | Elongation (%) | Density (g/cm3) | BET Surface Area (m2/g) | BJH Pore Volume (cm3/g) | BJH Pore Size (nm) | Elemental Composition | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Al | Ca | Cl | ||||||||
| Al2O3 | 100 | - | - | - | 217.1 | 0.21 | 3.27 | 44.4 | 0.013 | - |
| Nylon 6 | 0 | 8.6 0.4 | 15.5 1.5 | 1.02 0.05 | 7.0 | 0.02 | 5.42 | - | 0.009 | 0.083 |
| ANC 10 | 10 | 5.4 0.7 | 9.9 0.9 | 1.54 0.04 | 84.7 | 0.07 | 3.45 | 11.2 | 0.044 | 0.076 |
| ANC 20 | 20 | 3.8 0.9 | 6.8 0.2 | 1.63 0.03 | 125.8 | 0.11 | 3.30 | 23.2 | 0.062 | 0.061 |
| ANC 30 | 30 | 1.9 0.3 | 3.8 0.1 | 1.67 0.05 | 151.2 | 0.13 | 3.25 | 32.3 | 0.103 | 0.038 |
| Isotherm Models | Parameter | ANC 10 | ANC 20 | ANC 30 |
|---|---|---|---|---|
| Langmuir | KL | 0.008 | 0.014 | 0.015 |
| qm (mg/g) | 54.0 | 63.0 | 78.7 | |
| R2 | 0.835 | 0.878 | 0.873 | |
| Freundlich | KF | 1.887 | 2.975 | 3.792 |
| n | 1.954 | 1.990 | 2.013 | |
| R2 | 0.972 | 0.975 | 0.955 | |
| Pseudo-first-order | K1 | 0.046 | 0.050 | 0.071 |
| q1 (mg/g) | 5.2 | 5.5 | 6.1 | |
| R2 | 0.982 | 0.983 | 0.987 | |
| Pseudo-second-order | K2 | 0.02 | 0.01 | 0.01 |
| q2 (mg/g) | 6.55 | 8.00 | 9.64 | |
| R2 | 0.997 | 0.996 | 0.997 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phan, N.T.T.; Sato, M.; Kobayashi, T. Silica Scaling Inhibition in Water Treatment Process Using Fibrous Al2O3-Nylon 6 Adsorbents. Fibers 2024, 12, 11. https://doi.org/10.3390/fib12010011
Phan NTT, Sato M, Kobayashi T. Silica Scaling Inhibition in Water Treatment Process Using Fibrous Al2O3-Nylon 6 Adsorbents. Fibers. 2024; 12(1):11. https://doi.org/10.3390/fib12010011
Chicago/Turabian StylePhan, Ngan Thi Thu, Minehiko Sato, and Takaomi Kobayashi. 2024. "Silica Scaling Inhibition in Water Treatment Process Using Fibrous Al2O3-Nylon 6 Adsorbents" Fibers 12, no. 1: 11. https://doi.org/10.3390/fib12010011
APA StylePhan, N. T. T., Sato, M., & Kobayashi, T. (2024). Silica Scaling Inhibition in Water Treatment Process Using Fibrous Al2O3-Nylon 6 Adsorbents. Fibers, 12(1), 11. https://doi.org/10.3390/fib12010011