Reaction Spinning Titanium Dioxide Particle-Coated Carbon Fiber for Photoelectric Energy Conversion
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
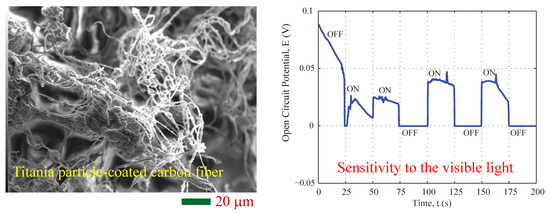
3.1. Morphology and Composition
3.2. Photovoltaic Property
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huang, Z.M.; Zhang, Y.Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Composites Sci. Tech. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Park, J.Y.; Lee, I.H. Characterization and Morphology of Prepared Titanium Dioxide Nanofibers by Electrospinning. J. Nanosci. Nanotech. 2010, 10, 3402–3405. [Google Scholar] [CrossRef]
- Caratao, B.; Carneiro, E.; Sa, P.; Almeida, B.; Carvalho, S. Properties of electrospun TiO2 nanofibers. J. Nanotech. 2014, 2014. [Google Scholar] [CrossRef]
- Zhao, L.R.; Jang, B.Z.; Zhou, J.L. Effect of polymeric precursors on properties of semiconducting carbon/carbon composites. J. Mater. Sci. 1998, 33, 1809–1817. [Google Scholar] [CrossRef]
- Gan, Y.X.; Draper, C.W.; Gan, J.B. Carbon nanofiber network made by electrohydrodynamic casting immiscible fluids. Mater. Today Comm. 2017, 13, 248–254. [Google Scholar] [CrossRef]
- Gan, Y.X.; Chen, A.D.; Gan, J.B.; Anderson, K.R. Electrohydrodynamic casting bismuth telluride micro particle loaded carbon nanofiber composite material with multiple sensing functions. J. Micro Nano-Manuf. 2018, 6. [Google Scholar] [CrossRef]
- Gan, Y.X.; Chen, A.D.; Gan, R.N.; Hamdan, A.S. Energy conversion behaviors of antimony telluride particle loaded partially carbonized nanofiber composite mat manufactured by electrohydrodynamic casting. Microelectron. Eng. 2017, 181, 16–21. [Google Scholar] [CrossRef]
- Gan, Y.X. A review of electrohydrodynamic casting polymer composites for energy conversions. AIMS Mater. Sci. 2018, 5, 206–225. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Tang, Z.; Bolong, N.; Saad, I. Response surface modeling of electrospinning parameters on titanium oxide nanofibers diameter: A Box-Behnken Design (BBD). Adv. Sci. Lett. 2017, 23, 11237–11241. [Google Scholar] [CrossRef]
- Singh, E.; Nalwa, H.S. Graphene-based dye-sensitized solar cells: A review. Sci. Adv. Mater. 2015, 7, 1863–1912. [Google Scholar] [CrossRef]
- Uddin, M.J.; Dickens, T.; Yan, J.; Chirayath, R.; Olawale, D.O.; Okoli, O.I. Solid state dye-sensitized photovoltaic micro-wires (DSPMs) with carbon nanotubes yarns as counter electrode: Synthesis and characterization. Solar Energy Mater. Solar Cells 2013, 108, 65–69. [Google Scholar] [CrossRef]
- Chronakis, I.S. Novel nanocomposites and nanoceramics based on polymer nanofibers using electrospinning process—A review. J. Mater. Proc. Tech. 2005, 167, 283–293. [Google Scholar] [CrossRef]
- Jalili, R.; Morshed, M.; Ravandi, S.A.H. Fundamental parameters affecting electrospinning of PAN nanofibers as uniaxially aligned fibers. J. Appl. Polym. Sci. 2006, 101, 4350–4357. [Google Scholar] [CrossRef]
- Watthanaarun, J.; Pavarajarn, V.; Supaphol, P. Titanium (IV) oxide nanofibers by combined sol-gel and electrospinning techniques: Preliminary report on effects of preparation conditions and secondary metal dopant. Sci. Tech. Adv. Mater. 2005, 6, 240–245. [Google Scholar] [CrossRef]
- Nuansing, W.; Ninmuang, S.; Jarenboon, W.; Maensiri, S.; Seraphin, S. Structural characterization and morphology of electrospun TiO2 nanofibers. Mater. Sci. Eng. B 2006, 131, 147–155. [Google Scholar] [CrossRef]
- Wijanarko, T.A.W.; Kusumaatmaja, A.; Roto, C.; Triyana, K. Effect of heat treatment on morphology and crystallinity of electrospun polyvinyl alcohol nanofibers. AIP Conf. Proc. 2016, 1755. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.Y.; Lim, J.H.; Myung, N.V. Optimization of thermoelectric properties of p-type AgSbTe2 thin films via electrochemical synthesis. Electrochim. Acta 2016, 196, 579–586. [Google Scholar] [CrossRef]







| Type of EDS | Locations for Analysis | Carbon | Oxygen | Titanium |
|---|---|---|---|---|
| area mapping | the entire region in Figure 4a | 54.59% | 28.40% | 17.01% |
| spot analysis | spot 001 shown in Figure 5a | 54.43% | 30.91% | 14.66% |
| spot analysis | spot 001 shown in Figure 5a | 34.57% | 33.97% | 31.47% |
| spot analysis | spot 001 shown in Figure 5a | 37.82% | 42.42% | 19.77% |
| Locations for Analysis | Carbon | Oxygen | Titanium |
|---|---|---|---|
| coated fibers | 49.42% | 34.23% | 16.21% |
| oxide clusters | 38.11% | 37.86% | 23.98% |
| Locations for Analysis | Carbon | Oxygen | Titanium |
|---|---|---|---|
| coated fibers | 9.07% | 7.75% | 6.24% |
| oxide clusters | 8.55% | 7.08% | 8.35% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, L.; Wei, X.; Martinez, J.P.; Yu, C.; Panahi, N.; Gan, J.B.; Zhang, Y.; Gan, Y.X. Reaction Spinning Titanium Dioxide Particle-Coated Carbon Fiber for Photoelectric Energy Conversion. Fibers 2019, 7, 49. https://doi.org/10.3390/fib7050049
Yuan L, Wei X, Martinez JP, Yu C, Panahi N, Gan JB, Zhang Y, Gan YX. Reaction Spinning Titanium Dioxide Particle-Coated Carbon Fiber for Photoelectric Energy Conversion. Fibers. 2019; 7(5):49. https://doi.org/10.3390/fib7050049
Chicago/Turabian StyleYuan, Leonardo, Xupeng Wei, Jenny P. Martinez, Christina Yu, Niousha Panahi, Jeremy B. Gan, Yongping Zhang, and Yong X. Gan. 2019. "Reaction Spinning Titanium Dioxide Particle-Coated Carbon Fiber for Photoelectric Energy Conversion" Fibers 7, no. 5: 49. https://doi.org/10.3390/fib7050049
APA StyleYuan, L., Wei, X., Martinez, J. P., Yu, C., Panahi, N., Gan, J. B., Zhang, Y., & Gan, Y. X. (2019). Reaction Spinning Titanium Dioxide Particle-Coated Carbon Fiber for Photoelectric Energy Conversion. Fibers, 7(5), 49. https://doi.org/10.3390/fib7050049