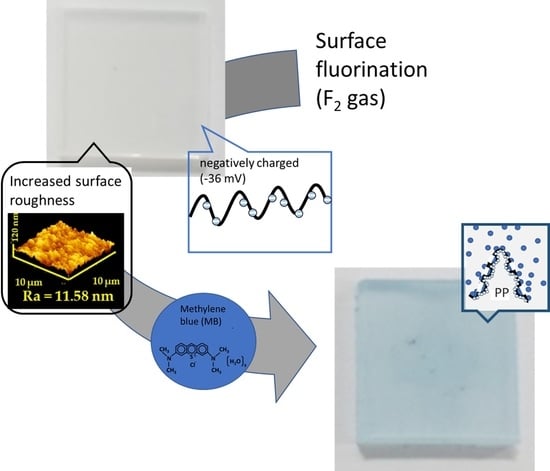
Improving the Dyeing of Polypropylene by Surface Fluorination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Surface Fluorination of Polycarbonate
2.2. Material Characterization
2.3. Dye Staining of Polypropylene
3. Results and Discussion
3.1. Effects of Fluorination on the Surface Morphology
3.2. Effects of Fluorination on the Surface Composition and Structure
3.3. Dyeing of Surface-Modified PP Plates
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anastasiadis, S.H. Development of functional polymer surfaces with controlled wettability. Langmuir 2013, 29, 9277–9290. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.G.; EL-Mahdy, A.F.M.; Meng, T.S.; Samy, M.M.; Kuo, S.W. Multifunctional hypercrosslinked porous organic polymers based on tetraphenylethene and triphenylamine derivatives for high-performance dye adsorption and supercapacitor. Polymers 2020, 12, 2426. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.G.; Tsai, M.Y.; Wang, C.F.; Huang, C.F.; Danko, M.; Dai, L.; Chen, T.; Kuo, S.W. Multifunctional polyhedral oligomeric silsesquioxane (POSS) based hybrid porous materials for CO2 uptake and iodine adsorption. Polymers 2021, 13, 221. [Google Scholar] [CrossRef] [PubMed]
- Hirai, S.; Phanthong, P.; Okubo, H.; Yao, S. Enhancement of the Surface Properties on Polypropylene Film Using Side-Chain Crystalline Block Copolymers. Polymers 2020, 12, 2736. [Google Scholar] [CrossRef]
- Hoff, A.; Jacobsson, S. Thermal oxidation of polypropylene in the temperature range of 120–280 °C. J. Appl. Polym. Sci. 1984, 29, 465–480. [Google Scholar] [CrossRef]
- Thakur, V.K.; Vennerberg, D.; Kessler, M.R. Green Aqueous Surface Modification of Polypropylene for Novel Polymer Nanocomposites. ACS Appl. Mater. Interfaces 2014, 6, 9349–9356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akrman, J.; Prikryl, J. Dyeing Behavior of Polypropylene Blend Fiber. I. Kinetic and Thermodynamic Parameters of the Dyeing System. J. Appl. Polym. Sci. 1996, 62, 235–240. [Google Scholar] [CrossRef]
- Tengsuwan, S.; Ohshima, M. Supercritical carbon dioxide-assisted electroless nickel plating on polypropylene—The effect of copolymer blend morphology on metal–polymer adhesion. J. Supercrit. Fluids 2014, 85, 123–134. [Google Scholar] [CrossRef]
- Shahidi, S.; Ghoranneviss, M.; Moazzenchi, B. Effect of using cold plasma on dyeing properties of polypropylene fabrics. Fibers Polym. 2007, 8, 123–129. [Google Scholar] [CrossRef]
- Burkinshaw, S.M.; Froehling, P.E.; Mignanelli, M. The effect of hyperbranched polymers on the dyeing of polypropylene fibres. Dye. Pigment. 2002, 53, 229–235. [Google Scholar] [CrossRef]
- Hachim, D.; Brown, B.N. Surface modification of polypropylene for enhanced layer by layer deposition of polyelectrolytes. J. Biomed. Mater. Res. A. 2018, 106, 2078–2085. [Google Scholar] [CrossRef] [PubMed]
- Walzak, M.J.; Flynn, S.; Foerch, R.; Hill, J.M. UV and ozone treatment of polypropylene and poly (ethylene terephthalate). J. Adhes. Sci. Technol. 1995, 9, 1229–1248. [Google Scholar] [CrossRef]
- Tanaka, S.; Naganuma, Y.; Kato, C.; Horie, K. Surface modification of vinyl polymers by vacuum ultraviolet light irradiation. J. Photopolym. Sci. Technol. 2003, 16, 165–170. [Google Scholar] [CrossRef] [Green Version]
- Strobe, M.; Jones, V.; Lyons, C.S.; Ulsh, M.; Kushner, M.J.; Dorai, R.; Branch, M.C. A Comparison of Corona-Treated and Flame-Treated Polypropylene Films. Plasmas Polym. 2003, 8, 61–95. [Google Scholar] [CrossRef]
- Blais, P.; Carlsson, D.J.; Csullog, G.W.; Wiles, D.M. The Chromic Acid Etching of Polyolefin Surfaces and Adhesive Bonding. J. Colloid Interface Sci. 1974, 47, 636–649. [Google Scholar] [CrossRef]
- Apel, P.Y.; Orelovich, O.L. Etching of submicron pores in thin polypropylene films irradiated with heavy ions. Nucl. Tracks Radiat. Meas. 1991, 19, 25–28. [Google Scholar] [CrossRef]
- Kharitonov, A.P.; Kharitonova, L.N. Surface modification of polymers by direct fluorination: A convenient approach to improve commercial properties of polymeric articles. Pure Appl. Chem. 2009, 81, 451–471. [Google Scholar] [CrossRef]
- Kirk, S.; Strobel, M.; Lee, C.; Pachuta, S.J.; Prokosch, M.; Lechuga, H.; Jones, M.E.; Lyons, C.S.; Degner, S.; Yang, Y.; et al. Fluorine plasma treatments of polypropylene films, 1-surface characterization. Plasma Process. Polym. 2010, 7, 107–122. [Google Scholar] [CrossRef]
- Kim, J.H.; Namie, M.; Yonezawa, S. Enhanced adhesion between polyethylene terephthalate and metal film by surface fluorination. Comp. Comm. 2018, 10, 205–208. [Google Scholar] [CrossRef]
- Kim, J.H.; Mishina, T.; Namie, M.; Nishimura, F.; Yonezawa, S. Effects of surface fluorination on the dyeing of polycarbonate (PC) resin. J. Coat. Technol. Res. 2021, 19, 617–624. [Google Scholar] [CrossRef]
- Geleji, F.; Selim, B.; Szabo, K. Pigmentation of polypropylene fibers. Faserforsch. Text. 1965, 16, 395–400. [Google Scholar]
- Assmann, K.; Schrenk, V. Develops new vehicle for dyeing polypropylene fibers. Int. Fiber J. 1997, 12, 44A. [Google Scholar]
- Kim, J.H.; Umeda, H.; Ohe, M.; Yonezawa, S.; Takashima, M. Preparation of pure LiPF6 using fluorine gas at room temperature. Chem. Lett. 2011, 40, 360–361. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Minbuta, S.; Matsui, K. Adsorption of the cationic dye methylene blue on anodic porous alumina in sodium dodecyl sulfate solutions. Langmuir 2020, 36, 4592–4599. [Google Scholar] [CrossRef]
- Fumoto, I. Studies on dyeing of polyolefins. (1) Dyeing properties of fluorinated polypropylene fiber. Seni Gakkaishi 1965, 21, 590–597. [Google Scholar] [CrossRef] [Green Version]










| Sample | F2 Gas Pressure | Reactionn Temp. | Reactionn Time |
|---|---|---|---|
| (Torr) | (°C) | (Min.) | |
| Untreated | - | - | - |
| F-10 | 10 | 25 °C | 60 |
| F-100 | 100 | ||
| F-380 | 380 |
| Sample Name | Elemental Composition Contents (%) | ||
|---|---|---|---|
| C | O | F | |
| untreated | 94.52 | 5.45 | 0.03 |
| F-10 | 35.14 | 4.64 | 60.22 |
| F-100 | 29.78 | 3.05 | 67.17 |
| F-380 | 30.50 | 3.18 | 66.32 |
| Sample Name | Elemental Composition Contents (%) | |||
|---|---|---|---|---|
| C | O | F | S | |
| Untreated | 85.05 | 12.66 | 1.18 | 1.11 |
| F-10 | 62.79 | 15.10 | 19.14 | 2.97 |
| F-100 | 55.65 | 12.94 | 29.07 | 2.34 |
| F-380 | 52.88 | 10.71 | 34.34 | 2.07 |
| Sample Name | Elemental Composition Contents (%) | |||
|---|---|---|---|---|
| C | O | F | S | |
| Untreated | 89.35 | 9.27 | 1.27 | 0.11 |
| F-10 | 38.87 | 7.71 | 53.18 | 0.24 |
| F-100 | 42.52 | 6.61 | 50.75 | 0.12 |
| F-380 | 33.73 | 4.36 | 61.68 | 0.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Namie, M.; Kim, J.-H.; Yonezawa, S. Improving the Dyeing of Polypropylene by Surface Fluorination. Colorants 2022, 1, 121-131. https://doi.org/10.3390/colorants1020008
Namie M, Kim J-H, Yonezawa S. Improving the Dyeing of Polypropylene by Surface Fluorination. Colorants. 2022; 1(2):121-131. https://doi.org/10.3390/colorants1020008
Chicago/Turabian StyleNamie, Masanari, Jae-Ho Kim, and Susumu Yonezawa. 2022. "Improving the Dyeing of Polypropylene by Surface Fluorination" Colorants 1, no. 2: 121-131. https://doi.org/10.3390/colorants1020008
APA StyleNamie, M., Kim, J. -H., & Yonezawa, S. (2022). Improving the Dyeing of Polypropylene by Surface Fluorination. Colorants, 1(2), 121-131. https://doi.org/10.3390/colorants1020008