A Predictive Model for Severe COVID-19 in the Medicare Population: A Tool for Prioritizing Primary and Booster COVID-19 Vaccination
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources
2.2. Dependent and Independent Variables
2.3. Statistical Analysis, Variable Selection, and Risk Model
2.4. Population-Level COVID-19 Hospitalization Risk Mapping
3. Results
3.1. Study Population Characteristics
3.2. Individual Predictors
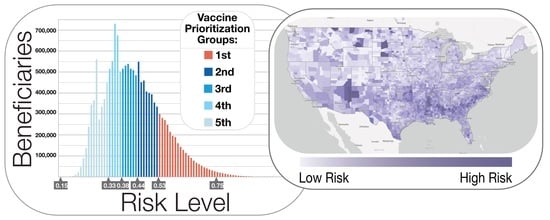
3.3. Population Risk Mapping and Stratification
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Department of Defense Disclaimer
Conflicts of Interest
References
- Oliver, S. Overview of Vaccine Equity and Prioritization Frameworks ACIP Meeting September 22, 2020. Centers for Disease Control and Prevention ACIP Covid-19 Vaccines Work Group; 2020. Available online: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2020-09/COVID-06-Oliver.pdf (accessed on 19 October 2021).
- Centers for Disease Control and Prevention. COVID-19 Mortality Overview. 2021. Available online: https://www.cdc.gov/nchs/covid19/mortality-overview.htm (accessed on 20 October 2021).
- Centers for Disease Control and Prevention. COVID-19 Information for Specific Groups of People updated April 20, 2021. 2021. Available online: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/index.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fneed-extra-precautions%2Fpeople-at-increased-risk.html (accessed on 19 October 2021).
- National Academies of Sciences, Engineering, and Medicine. National Academies Release Framework for Equitable Allocation of a COVID-19 Vaccine for Adoption by HHS, State, Tribal, Local, and Territorial Authorities. ScienceDaily. 2020. Available online: wwwsciencedaily.com/releases/2020/10/201002111729.htm (accessed on 19 October 2021).
- Centers for Disease Control and Prevention. CDC SVI 2018 Documentation. 2021. Available online: https://svi.cdc.gov/Documents/Data/2018_SVI_Data/SVI2018Documentation.pdf (accessed on 19 October 2021).
- Surgo Ventures: The U.S. Covid Community Vulnerability Index (CCVI). Vulnerability—How Well a Community Handles the Repercussions of a COVID-19 Outbreak—Matters. 2021. Available online: https://precisionforcovid.org/ccvi (accessed on 19 October 2021).
- Dooling, K. Phase 1 Allocation COVID-19 Vaccine: Work Group Considerations ACIP Meeting September 22, 2020. Centers for Disease Control and Prevention ACIP Covid-19 Vaccines Work Group 2020. Available online: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2020-09/COVID-07-Dooling.pdf (accessed on 19 October 2021).
- Centers for Disease Control and Prevention. Percent of U.S. Adults 55 and Over with Chronic Conditions. 2021. Available online: https://www.cdc.gov/nchs/health_policy/adult_chronic_conditions.htm (accessed on 19 October 2021).
- Dooling, K. Phased Allocation of COVID-19 Vaccines ACIP Meeting November 23, 2020. Centers for Disease Control and Prevention ACIP Covid-19 Vaccines Work Group 2020. Available online: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2020-11/COVID-04-Dooling.pdf (accessed on 19 October 2021).
- Centers for Disease Control and Prevention. People with Certain Medical Conditions. 2021. Available online: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html (accessed on 19 October 2021).
- CDC COVID-19 Response Team. Preliminary Estimates of the Prevalence of Selected Underlying Health Conditions Among Patients with Coronavirus Disease 2019—United States, February 12–March 18, 2020. Morb. Mortal. Wkly. Rep. 2020, 69, 382–386. [Google Scholar] [CrossRef]
- Lithander, F.E.; Neumann, S.; Tenison, E.; Lloyd, K.; Welsh, T.J.; Rodrigues, J.C.L.; Higgins, J.P.T.; Scourfield, L.; Christensen, H.; Haunton, V.J.; et al. COVID-19 in older people: A rapid clinical review. Age Ageing 2020, 49, 501–515. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Kim, L.; Whitaker, M.; O’Halloran, A.; Cummings, C.; Holstein, R.; Prill, M.; Chai, S.J.; Kirley, P.D.; Alden, N.B.; et al. Hospitalization Rates and Characteristics of Patients Hospitalized with Laboratory-Confirmed Coronavirus Disease 2019—COVID-NET, 14 States, March 1–30, 2020. Morb. Mortal. Wkly. Rep. 2020, 69, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Centers for Medicare and Medicaid Services. Preliminary Medicare COVID-19 Data Snapshot. 2021. Available online: https://www.cms.gov/research-statistics-data-systems/preliminary-medicare-covid-19-data-snapshot (accessed on 19 October 2021).
- Chang, M.-H.; Mooneshinghe, R.; Truman, B.I. Racial and ethnic differences in COVID-19 hospitalizations by metropolitan status among Medicare beneficiaries, 1 January–31 December 2020. J. Public Health 2021. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services Office of Inspector General. Medicare Beneficiaries Hospitalized with COVID-19 Experienced a Wide Range of Serious, Complex Conditions. 2021. Available online: https://oig.hhs.gov/oei/reports/OEI-02-20-00410.pdf (accessed on 27 October 2021).
- Bosworth, A.; Finegold, K.; Samsong, L.W.; Sheingold, S.; Tarazi, W.; Zuckerman, R.; HHS Assistant Secretary for Planning and Evaluation Office of Health Policy. Risk of Covid-19 Infection, Hospitalization, and Death in Fee-For-Service Medicare. 2021. Available online: https://aspe.hhs.gov/sites/default/files/private/pdf/265271/risk-score-issue-brief.pdf (accessed on 26 October 2021).
- Lurie, N.; Experton, B. How to Leverage the Medicare Program for a COVID-19 Vaccination Campaign. JAMA 2021, 325, 2–22. [Google Scholar] [CrossRef] [PubMed]
- AI in Defense DoD’s Artificial Intelligence Blog. The JAIC Forges Ahead. 2020. Available online: https://www.ai.mil/blog_05_20_20-the_jaic_forges_ahead.html (accessed on 19 October 2021).
- HUD Office of Policy Development and Research. HUD USPS Zip Code Q2 2020 Crosswalk Files. 2020. Available online: https://www.huduser.gov/portal/datasets/usps_crosswalk.html (accessed on 19 October 2021).
- R Foundation for Statistical Computing. The R Project for Statistical Computing. 2021. Available online: https://www.r-project.org/ (accessed on 19 October 2021).
- Harrell, F.E. Regression Modelling Strategies. CDC Virtual Course 20–23 September 2021. Available online: https://hbiostat.org/doc/rms.pdf (accessed on 19 October 2021).
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.C.; Muller, M. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.; Hastie, T.; Tibshirani, R. Regularization Paths for Generalized Linear Models via Coordinate Descent. J. Stat. Softw. 2010, 33, 1–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedregosa, F.; Varoquax, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weis, R.; Dubourg, V.; et al. Scikit-learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Czaja, C.A.; Miller, L.; Alden, N.; Wald, H.L.; Cummings, C.N.; Rolfes, M.A.; Anderson, E.J.; Bennett, N.M.; Billing, L.M.; Chai, S.J.; et al. Age-Related Differences in Hospitalization Rates, Clinical Presentation, and Outcomes Among Older Adults Hospitalized with Influenza-U.S. Influenza Hospitalization Surveillance Network (FluSurv-NET). Open Forum Infect. Dis. 2019, 6, ofz225. [Google Scholar] [CrossRef] [PubMed]
- Moyo, P.; Zullo, A.R.; McConeghy, K.W.; Bosco, E.; van Aalst, R.; Chit, A.; Gravenstein, S. Risk factors for pneumonia and influenza hospitalizations in long-term care facility residents: A retrospective cohort study. BMC Geriatr. 2020, 20, 47. [Google Scholar] [CrossRef] [PubMed]
- Centers for Medicare and Medicaid Services. Chronic Conditions Charts: 2018. 2018. Available online: https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Chronic-Conditions/Chartbook_Charts (accessed on 19 October 2021).
- Centers for Disease Control and Prevention. Risk for COVID-19 Infection, Hospitalization, and Death By Age Group. 2021. Available online: https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-age.html (accessed on 19 October 2021).
- Centers for Disease Control and Prevention. Underlying Medical Conditions Associated with Higher Risk for Severe COVID-19: Information for Healthcare Providers. 2021. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html (accessed on 26 October 2021).
- Acosta, A.M.; Garg, S.; Pham, H.; Whitaker, M.; Anglin, O.; O’Halloran, A.; Milucky, J.; Patel, K.; Taylor, C.; Wortham, J.; et al. Racial and Ethnic Disparities in Rates of COVID-19-Associated Hospitalization, Intensive Care Unit Admission, and In-Hospital Death in the United States from March 2020 to February 2021. JAMA Netw. Open 2021, 4, e2130479. [Google Scholar] [CrossRef] [PubMed]
- Azar, K.M.J.; Shen, Z.; Romanelli, R.J.; Lockhart, S.H.; Smits, K.; Robinson, S.; Brown, S.; Pressman, A.R. Disparities In Outcomes Among COVID-19 Patients In A Large Health Care System In California. Health Aff. 2020, 39, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Palaiodimos, L.; Kokkinidis, D.G.; Li, W.; Karamanis, D.; Ognibene, J.; Arora, S.; Southern, W.N.; Mantzoros, C.S. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx, New York. Metabolism 2020, 108, 154262. [Google Scholar] [CrossRef] [PubMed]
- Experton, B.; Li, Z.; Branch, L.G.; Ozminkowski, R.J.; Mellon-Lacey, D.M. The impact of payor/provider type on health care use and expenditures among the frail elderly. Am. J. Public Health 1997, 87, 210–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; the Northwell COVID-19 Research Consortium; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Henry, B.M. Chronic obstructive pulmonary disease is associated with severe coronavirus disease 2019 (COVID-19). Respir. Med. 2020, 167, 105941. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zeng, M.; Wang, H.; Qin, C.; Hou, H.Y.; Sun, Z.Y.; Xu, S.P.; Wang, G.P.; Guo, C.L.; Deng, Y.K.; et al. Distinct effects of asthma and COPD comorbidity on disease expression and outcome in patients with COVID-19. Allergy 2021, 76, 483–496. [Google Scholar] [CrossRef] [PubMed]
- Oliver, S. Considerations for booster doses of COVID-19 vaccines ACIP Meeting August 13, 2021. Centers for Disease Control and Prevention; 2021. Available online: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-08-13/05-COVID-Oliver-508.pdf (accessed on 19 October 2021).
- Centers for Disease Control and Prevention. U.S. Influenza Surveillance: Purpose and Methods. Available online: https://www.cdc.gov/flu/weekly/overview.htm (accessed on 27 October 2021).
- Centers for Disease Control and Prevention COVID-NET. Available online: https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covid-net/purpose-methods.html (accessed on 27 October 2021).





| Variable | Non-Covid-19 Cases † | Covid-19 Outpatients ‡ | Covid-19 Hospitalized ¶ | Covid-19 Deaths # | |||||
|---|---|---|---|---|---|---|---|---|---|
| Total | 14,592,352 | 421,575 | 345,111 | 135,567 | |||||
| Age | Median | (IQR) | Median | (IQR) | Median | (IQR) | Median | (IQR) | |
| Age | 73 | (67–80) ✦✦✦ | 73 | (68–81) | 76 | (68–84) ✦✦✦ | 82 | (73–89) ✦✦✦ | |
| % | (no.) | % | (no.) | % | (no.) | % | (no.) | ||
| Under 65 | 15.20% | (2,221,832) *** | 14.60% | (61,446) | 14.30% | (49,202) *** | 7.40% | (10,062) *** | |
| From 65 to 74 | 41.50% | (6,061,460) *** | 40.40% | (170,315) | 32.10% | (110,873) *** | 21.50% | (29,161) *** | |
| From 75 to 84 | 28.80% | (4,208,784) *** | 27.00% | (113,939) | 30.80% | (106,170) *** | 31.20% | (42,248) *** | |
| Over 85 | 14.40% | (2,100,276) *** | 18.00% | (75,875) | 22.90% | (78,866) *** | 39.90% | (54,096) *** | |
| Sex | % | (no.) | % | (no.) | % | (no.) | % | (no.) | |
| Male | 43.40% | (6,330,698) | 40.50% | (170,855) | 48.50% | (167,259) *** | 48.40% | (65,679) *** | |
| Female | 56.60% | (8,261,652) | 59.50% | (250,720) | 51.50% | (177,852) *** | 51.60% | (69,888) *** | |
| Race | % | (no.) | % | (no.) | % | (no.) | % | (no.) | |
| North American Native | 0.60% | (90,876) *** | 0.50% | (2285) | 1.00% | (3410) *** | 0.90% | (1171) *** | |
| Black | 9.60% | (1,394,033) *** | 12.50% | (52,738) | 18.10% | (62,302) *** | 16.50% | (22,338) *** | |
| Hispanic | 2.00% | (293,861) *** | 3.80% | (15,971) | 4.70% | (16,125) *** | 4.20% | (5729) *** | |
| Asian | 1.90% | (277,030) *** | 2.10% | (8728) | 2.30% | (7857) *** | 2.40% | (3248) *** | |
| White | 82.20% | (12,002,137) *** | 77.20% | (325,301) | 70.80% | (244,233) *** | 73.40% | (99,458) *** | |
| Other | 1.60% | (227,572) * | 1.70% | (7057) | 1.70% | (5890) | 1.60% | (2223) | |
| Unknown | 2.10% | (306,843) *** | 2.30% | (9495) | 1.50% | (5294) *** | 1.00% | (1400) *** | |
| Socio-economic (SVI) Variable Quartiles | % | (no.) | % | (no.) | % | (no.) | % | (no.) | |
| Lowest Income (EPL_PCI) | 11.20% | (1,635,128) *** | 13.70% | (57,813) | 17.60% | (60,773) *** | 16.80% | (22,732) *** | |
| Most Crowded Housing (EPL_CROWD) | 10.00% | (1,452,010) *** | 16.20% | (68,406) | 16.50% | (56,957) ** | 16.60% | (22,475) *** | |
| Highest Multi-unit Housing (EPL_MUNIT) | 11.70% | (1,700,037) *** | 17.70% | (74,648) | 16.00% | (55,163) *** | 16.70% | (22,659) *** | |
| Highest Institutional Housing (EPL_GROUPQ) | 7.50% | (1,092,374) | 7.10% | (29,776) | 7.00% | (24,092) | 7.20% | (9731) *** | |
| % | (no.) | % | (no.) | % | (no.) | % | (no.) | ||
| Disabled | 24.80% | (3,619,734) *** | 26.00% | (109,502) | 27.40% | (94,554) *** | 21.80% | (29,522) *** | |
| Dual Medicare-Medicaid | 21.80% | (3,187,875) *** | 37.50% | (158,292) | 40.00% | (137,894) *** | 47.70% | (64,679) *** | |
| Prior Hospitalization | % | (no.) | % | (no.) | % | (no.) | % | (no.) | |
| 0 | 100.00% | (14,592,352) *** | 79.20% | (333,743) | 64.20% | (221,694) *** | 62.20% | (84,328) *** | |
| 1 or more | 0.00% | NA | 20.80% | (87,832) | 35.80% | (123,417) *** | 37.80% | (51,239) *** | |
| Clinical Variables | % | (no.) | % | (no.) | % | (no.) | % | (no.) | |
| ESRD | 1.80% | (260,810) *** | 2.10% | (8940) | 6.30% | (21,703) *** | 5.00% | (6814) *** | |
| Chronic Kidney Disease | 36.70% | (5,351,947) *** | 39.50% | (166,536) | 53.00% | (182,910) *** | 57.60% | (78,103) *** | |
| Pulmonary Fibrosis or HTN | 7.10% | (1,030,957) *** | 5.50% | (23,231) | 9.00% | (31,198) *** | 9.40% | (12,677) *** | |
| Chronic Liver Disease | 2.60% | (379,804) *** | 2.50% | (10,525) | 3.80% | (13,032) *** | 3.60% | (4901) *** | |
| COPD | 25.80% | (3,764,077) *** | 27.70% | (116,650) | 36.20% | (124,828) *** | 39.70% | (53,790) *** | |
| CHF | 24.40% | (3,553,556) *** | 29.70% | (125,223) | 40.70% | (140,534) *** | 48.20% | (65,366) *** | |
| Stroke/TIA | 13.50% | (1,968,280) *** | 17.90% | (75,473) | 22.30% | (77,087) *** | 28.40% | (38,437) *** | |
| Diabetes | 37.30% | (5,441,056) *** | 44.50% | (187,722) | 52.70% | (182,045) *** | 55.30% | (75,020) *** | |
| Hypertension | 76.10% | (11,107,034) *** | 79.10% | (333,506) | 84.80% | (292,744) *** | 88.70% | (120,301) *** | |
| Acute MI | 0.90% | (130,435) *** | 0.80% | (3327) | 1.50% | (5101) *** | 1.60% | (2160) *** | |
| Ischemic Heart Disease | 44.00% | (6,427,825) *** | 48.90% | (206,139) | 57.60% | (198,625) *** | 64.40% | (87,267) *** | |
| Asthma | 16.20% | (2,360,423) *** | 17.50% | (73,567) | 19.60% | (67,752) *** | 18.70% | (25,301) *** | |
| Chemotherapy | 9.10% | (1,321,542) *** | 11.30% | (47,845) | 14.40% | (49,598) *** | 13.60% | (18,494) *** | |
| Obesity | 15.10% | (2,200,797) *** | 16.30% | (68,926) | 16.70% | (57,706) *** | 11.90% | (16,076) *** | |
| Morbid Obesity | 8.70% | (1,264,568) *** | 9.80% | (41,133) | 13.90% | (48,083) *** | 9.60% | (13,013) *** | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Experton, B.; Tetteh, H.A.; Lurie, N.; Walker, P.; Elena, A.; Hein, C.S.; Schwendiman, B.; Vincent, J.L.; Burrow, C.R. A Predictive Model for Severe COVID-19 in the Medicare Population: A Tool for Prioritizing Primary and Booster COVID-19 Vaccination. Biology 2021, 10, 1185. https://doi.org/10.3390/biology10111185
Experton B, Tetteh HA, Lurie N, Walker P, Elena A, Hein CS, Schwendiman B, Vincent JL, Burrow CR. A Predictive Model for Severe COVID-19 in the Medicare Population: A Tool for Prioritizing Primary and Booster COVID-19 Vaccination. Biology. 2021; 10(11):1185. https://doi.org/10.3390/biology10111185
Chicago/Turabian StyleExperton, Bettina, Hassan A. Tetteh, Nicole Lurie, Peter Walker, Adrien Elena, Christopher S. Hein, Blake Schwendiman, Justin L. Vincent, and Christopher R. Burrow. 2021. "A Predictive Model for Severe COVID-19 in the Medicare Population: A Tool for Prioritizing Primary and Booster COVID-19 Vaccination" Biology 10, no. 11: 1185. https://doi.org/10.3390/biology10111185
APA StyleExperton, B., Tetteh, H. A., Lurie, N., Walker, P., Elena, A., Hein, C. S., Schwendiman, B., Vincent, J. L., & Burrow, C. R. (2021). A Predictive Model for Severe COVID-19 in the Medicare Population: A Tool for Prioritizing Primary and Booster COVID-19 Vaccination. Biology, 10(11), 1185. https://doi.org/10.3390/biology10111185