Long-Term Aerobic Training Improves Mitochondrial and Antioxidant Function in the Liver of Wistar Rats Preventing Hepatic Age-Related Function Decline
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Groups
2.2. Exercise Training Program
2.3. Sample Collection
2.4. Oxidative Stress Evaluation
2.5. Mitochondrial Bioenergetics Evaluation
2.6. Mitochondrial Respiratory Activity
2.7. Statistical Analysis
3. Results
3.1. Mortality Rate, Body Weight and Food and Water Consumption
3.2. Lifelong Exercise, Chronological Age and Oxidative Stress
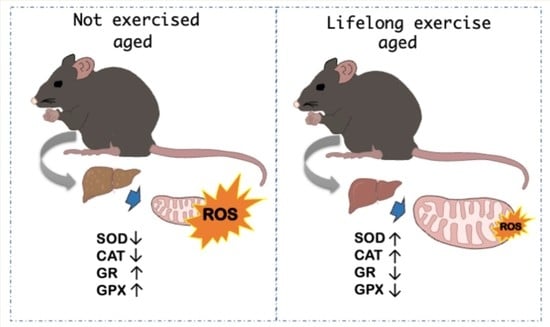
3.2.1. Antioxidant Enzymes
3.2.2. Biochemical Oxidative Stress Markers
3.2.3. Lifelong Exercise, Chronological Age and Mitochondrial Bioenergetics
3.2.4. Lifelong Exercise, Chronological Age and Mitochondrial Respiratory Capacity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Judge, S.; Leeuwenburgh, C. Cardiac Mitochondrial Bioenergetics, Oxidative Stress, and Aging. Am. J. Physiol.—Cell Physiol. 2007, 292, 1–2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harman, D. The Biologic Clock: The Mitochondria? J. Am. Geriatr. Soc. 1972, 20, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. Aging: A Theory Based on Free Radical and Radiation Chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radak, Z.; Chung, H.Y.; Koltai, E.; Taylor, A.W.; Goto, S. Exercise, Oxidative Stress and Hormesis. Ageing Res. Rev. 2008, 7, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Navarro, A.; Gomez, C.; López-Cepero, J.M.; Boveris, A. Beneficial Effects of Moderate Exercise on Mice Aging: Survival, Behavior, Oxidative Stress, and Mitochondrial Electron Transfer. Am. J. Physiol. Integr. Comp. Physiol. 2003, 286, R505–R511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gil del Valle, L. Oxidative Stress in Aging: Theoretical Outcomes and Clinical Evidences in Humans. Biomed. Aging Pathol. 2011, 1, 1–7. [Google Scholar] [CrossRef]
- Radák, Z.; Chung, H.Y.; Naito, H.; Takahashi, R.; Jung, K.J.; Kim, H.-J.; Goto, S. Age-Associated Increase in Oxidative Stress and Nuclear Factor ΚB Activation Are Attenuated in Rat Liver by Regular Exercise. FASEB J. 2004, 18, 749–750. [Google Scholar] [CrossRef]
- Nakanishi, K.; Sakakima, H.; Norimatsu, K.; Otsuka, S.; Takada, S.; Tani, A.; Kikuchi, K. Effect of Low-Intensity Motor Balance and Coordination Exercise on Cognitive Functions, Hippocampal Aβ Deposition, Neuronal Loss, Neuroinflammation, and Oxidative Stress in a Mouse Model of Alzheimer’s Disease. Exp. Neurol. 2021, 337, 113590. [Google Scholar] [CrossRef]
- Chae, C.H.; Shin, C.H.; Kim, H.T. The Combination of α-Lipoic Acid Supplementation and Aerobic Exercise Inhibits Lipid Peroxidation in Rat Skeletal Muscles. Nutr. Res. 2008, 28, 399–405. [Google Scholar] [CrossRef]
- Oh, S.; So, R.; Shida, T.; Matsuo, T.; Kim, B.; Akiyama, K.; Isobe, T.; Okamoto, Y.; Tanaka, K.; Shoda, J. High-Intensity Aerobic Exercise Improves Both Hepatic Fat Content and Stiffness in Sedentary Obese Men with Nonalcoholic Fatty Liver Disease. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Kvam, S.; Kleppe, C.L.; Nordhus, I.H.; Hovland, A. Exercise as a Treatment for Depression: A Meta-Analysis. J. Affect. Disord. 2016, 202, 67–86. [Google Scholar] [CrossRef] [PubMed]
- Wiklund, P. The Role of Physical Activity and Exercise in Obesity and Weight Management: Time for Critical Appraisal. J. Sport Health Sci. 2016, 5, 151–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peake, J.M.; Markworth, J.F.; Nosaka, K.; Raastad, T.; Wadley, G.D.; Coffey, V.G. Modulating Exercise-Induced Hormesis: Does Less Equal More? J. Appl. Physiol. 2015, 119, 172–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powers, S.K.; Jackson, M.J. Exercise-Induced Oxidative Stress: Cellular Mechanisms and Impact on Muscle Force Production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [CrossRef] [Green Version]
- Bishop, D.J.; Granata, C.; Eynon, N. Can We Optimise the Exercise Training Prescription to Maximise Improvements in Mitochondria Function and Content? Biochim. Biophys. Acta—Gen. Subj. 2014, 1840, 1266–1275. [Google Scholar] [CrossRef] [Green Version]
- Radak, Z.; Zhao, Z.; Koltai, E.; Ohno, H.; Atalay, M. Oxygen Consumption and Usage During Physical Exercise: The Balance Between Oxidative Stress and ROS-Dependent Adaptive Signaling. Antioxid. Redox Signal. 2013, 18, 1208–1246. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Cabrera, M.-C.; Domenech, E.; Viña, J. Moderate Exercise Is an Antioxidant: Upregulation of Antioxidant Genes by Training. Free Radic. Biol. Med. 2008, 44, 126–131. [Google Scholar] [CrossRef]
- Alessio, H.M.; Hagerman, A.E.; Fulkerson, B.K.; Ambrose, J.; Rice, R.E.; Wiley, R.L. Generation of Reactive Oxygen Species after Exhaustive Aerobic and Isometric Exercise. Med. Sci. Sports Exerc. 2000, 32, 1576–1581. [Google Scholar] [CrossRef]
- Dominiak, K.; Galganski, L.; Budzinska, A.; Woyda-Ploszczyca, A.; Zoladz, J.A.; Jarmuszkiewicz, W. Effects of Endurance Training on the Coenzyme Q Redox State in Rat Heart, Liver, and Brain at the Tissue and Mitochondrial Levels: Implications for Reactive Oxygen Species Formation and Respiratory Chain Remodeling. Int. J. Mol. Sci. 2022, 23, 896. [Google Scholar] [CrossRef]
- Merry, T.L.; Ristow, M. Mitohormesis in Exercise Training. Free Radic. Biol. Med. 2016, 98, 123–130. [Google Scholar] [CrossRef]
- Oliveira, A.N.; Hood, D.A. Exercise Is Mitochondrial Medicine for Muscle. Sport. Med. Health Sci. 2019, 1, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Steiner, J.L.; Murphy, E.A.; McClellan, J.L.; Carmichael, M.D.; Davis, J.M. Exercise Training Increases Mitochondrial Biogenesis in the Brain. J. Appl. Physiol. 2011, 111, 1066–1071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kacerovsky-Bielesz, G.; Chmelik, M.; Ling, C.; Pokan, R.; Szendroedi, J.; Farukuoye, M.; Kacerovsky, M.; Schmid, A.I.; Gruber, S.; Wolzt, M.; et al. Short-Term Exercise Training Does Not Stimulate Skeletal Muscle ATP Synthesis in Relatives of Humans with Type 2 Diabetes. Diabetes 2009, 58, 1333–1341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marshall, R.N.; Smeuninx, B.; Seabright, A.P.; Morgan, P.T.; Atherton, P.J.; Philp, A.; Breen, L. No Effect of Five Days of Bed Rest or Short-Term Resistance Exercise Prehabilitation on Markers of Skeletal Muscle Mitochondrial Content and Dynamics in Older Adults. Physiol. Rep. 2022, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.A.; Meers, G.M.; Linden, M.A.; Kearney, M.L.; Morris, E.M.; Thyfault, J.P.; Rector, R.S. Impact of Various Exercise Modalities on Hepatic Mitochondrial Function. Med. Sci. Sport. Exerc. 2014, 46, 1089–1097. [Google Scholar] [CrossRef] [Green Version]
- Marmett, B.; Nunes, R.B.; de Souza, K.S.; Dal Lago, P.; Rhoden, C.R. Aerobic Training Reduces Oxidative Stress in Skeletal Muscle of Rats Exposed to Air Pollution and Supplemented with Chromium Picolinate. Redox Rep. 2018, 23, 146–152. [Google Scholar] [CrossRef]
- Powers, S.K.; Nelson, W.B.; Hudson, M.B. Exercise-Induced Oxidative Stress in Humans: Cause and Consequences. Free Radic. Biol. Med. 2011, 51, 942–950. [Google Scholar] [CrossRef]
- Sun, L.; Shen, W.; Liu, Z.; Guan, S.; Liu, J.; Ding, S. Endurance Exercise Causes Mitochondrial and Oxidative Stress in Rat Liver: Effects of a Combination of Mitochondrial Targeting Nutrients. Life Sci. 2010, 86, 39–44. [Google Scholar] [CrossRef]
- Gonçalves, I.O.; Passos, E.; Rocha-Rodrigues, S.; Diogo, C.V.; Torrella, J.R.; Rizo, D.; Viscor, G.; Santos-Alves, E.; Marques-Aleixo, I.; Oliveira, P.J.; et al. Physical Exercise Prevents and Mitigates Non-Alcoholic Steatohepatitis-Induced Liver Mitochondrial Structural and Bioenergetics Impairments. Mitochondrion 2014, 15, 40–51. [Google Scholar] [CrossRef]
- Trefts, E.; Williams, A.S.; Wasserman, D.H. Exercise and the Regulation of Hepatic Metabolism. Prog. Mol. Biol. Transl. Sci. 2015, 135, 203–225. [Google Scholar] [CrossRef]
- Kakarla, P.; Kesireddy, S.; Christiaan, L. Exercise Training with Ageing Protects against Ethanol Induced Myocardial Glutathione Homeostasis. Free Radic. Res. 2008, 42, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Gornall, A.G.; Bardawill, C.J.; David, M.M. Determination of serum proteins by means of the biuret reaction. J. Biol. Chem. 1949, 177, 751–766. [Google Scholar] [CrossRef] [PubMed]
- Payá, M.; Halliwell, B.; Hoult, J.R.S. Interactions of a Series of Coumarins with Reactive Oxygen Species. Biochem. Pharmacol. 1992, 44, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, F.; Barros, A.I.R.N.A.; Silva, A.M.S. Interactions of a New 2-Styrylchromone with Mitochondrial Oxidative Phosphorylation. J. Biochem. Mol. Toxicol. 2002, 16, 220–226. [Google Scholar] [CrossRef]
- del Río, L.A.; Ortega, M.G.; López, A.L.; Gorgé, J.L. A More Sensitive Modification of the Catalase Assay with the Clark Oxygen Electrode. Application to the Kinetic Study of the Pea Leaf Enzyme. Anal. Biochem. 1977, 80, 409–415. [Google Scholar] [CrossRef]
- Félix, L.M.; Correia, F.; Pinto, P.A.; Campos, S.P.; Fernandes, T.; Videira, R.; Oliveira, M.M.; Peixoto, F.P.; Antunes, L.M. Propofol Affinity to Mitochondrial Membranes Does Not Alter Mitochondrial Function. Eur. J. Pharmacol. 2017, 803, 48–56. [Google Scholar] [CrossRef]
- Mannervik, B. Glutathione Peroxidase. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1985; Volume 113, pp. 490–495. ISBN 0076-6879. [Google Scholar]
- Carlberg, I.; Mannervik, B. Glutathione Reductase. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1985; Volume 113, pp. 484–490. ISBN 0076-6879. [Google Scholar]
- Oliveira, M.M.; Teixeira, J.C.; Vasconcelos-Nóbrega, C.; Felix, L.M.; Sardão, V.A.; Colaço, A.A.; Oliveira, P.A.; Peixoto, F.P. Mitochondrial and Liver Oxidative Stress Alterations Induced by N-Butyl-N-(4-Hydroxybutyl)Nitrosamine: Relevance for Hepatotoxicity. J. Appl. Toxicol. 2013, 33, 434–443. [Google Scholar] [CrossRef]
- Ottolenghi, A. Interaction of Ascorbic Acid and Mitochondrial Lipides. Arch. Biochem. Biophys. 1959, 79, 355–363. [Google Scholar] [CrossRef]
- Hissin, P.J.; Hilf, R. A Fluorometric Method for Determination of Oxidized and Reduced Glutathione in Tissues. Anal. Biochem. 1976, 74, 214–226. [Google Scholar] [CrossRef]
- Peixoto, F.; Vicente, J.; Madeira, V.M.C. A Comparative Study of Plant and Animal Mitochondria Exposed to Paraquat Reveals That Hydrogen Peroxide Is Not Related to the Observed Toxicity. Toxicol. Vitr. 2004, 18, 733–739. [Google Scholar] [CrossRef]
- Leek, B.T.; Mudaliar, S.R.D.; Henry, R.; Mathieu-Costello, O.; Richardson, R.S. Effect of Acute Exercise on Citrate Synthase Activity in Untrained and Trained Human Skeletal Muscle. Am. J. Physiol. - Regul. Integr. Comp. Physiol. 2001, 280, 441–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marosi, K.; Bori, Z.; Hart, N.; Sárga, L.; Koltai, E.; Radák, Z.; Nyakas, C. Long-Term Exercise Treatment Reduces Oxidative Stress in the Hippocampus of Aging Rats. Neuroscience 2012, 226, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Radak, Z.; Sasvári, M.; Nyakas, C.; Taylor, A.W.; Ohno, H.; Nakamoto, H.; Goto, S. Regular Training Modulates the Accumulation of Reactive Carbonyl Derivatives in Mitochondrial and Cytosolic Fractions of Rat Skeletal Muscle. Arch. Biochem. Biophys. 2000, 383, 114–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siu, P.M.; Donley, D.A.; Bryner, R.W.; Alway, S.E. Citrate Synthase Expression and Enzyme Activity after Endurance Training in Cardiac and Skeletal Muscles. J. Appl. Physiol. 2003, 94, 555–560. [Google Scholar] [CrossRef] [Green Version]
- Navarro, A.; Boveris, A. Rat Brain and Liver Mitochondria Develop Oxidative Stress and Lose Enzymatic Activities on Aging. Am. J. Physiol. Integr. Comp. Physiol. 2004, 287, 1244–1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duicu, O.M.; Mirica, S.N.; Gheorgheosu, D.E.; Privistirescu, A.I.; Fira-Mladinescu, O.; Muntean, D.M. Ageing-Induced Decrease in Cardiac Mitochondrial Function in Healthy Rats. Can. J. Physiol. Pharmacol. 2013, 91, 593–600. [Google Scholar] [CrossRef]
- Srividhya, R.; Zarkovic, K.; Stroser, M.; Waeg, G.; Zarkovic, N.; Kalaiselvi, P. Mitochondrial Alterations in Aging Rat Brain: Effective Role of (-)-Epigallo Catechin Gallate. Int. J. Dev. Neurosci. 2009, 27, 223–231. [Google Scholar] [CrossRef]
- Santos-Alves, E.; Marques-Aleixo, I.; Rizo-Roca, D.; Torrella, J.R.; Oliveira, P.J.; Magalhães, J.; Ascensão, A. Exercise Modulates Liver Cellular and Mitochondrial Proteins Related to Quality Control Signaling. Life Sci. 2015, 135, 124–130. [Google Scholar] [CrossRef]
- Delaval, E.; Perichon, M.; Friguet, B. Age-Related Impairment of Mitochondrial Matrix Aconitase and ATP-Stimulated Protease in Rat Liver and Heart. Eur. J. Biochem. 2004, 271, 4559–4564. [Google Scholar] [CrossRef]
- Gusdon, A.M.; Callio, J.; Distefano, G.; O’Doherty, R.M.; Goodpaster, B.H.; Coen, P.M.; Chu, C.T. Exercise Increases Mitochondrial Complex I Activity and DRP1 Expression in the Brains of Aged Mice. Exp. Gerontol. 2017, 90, 1–13. [Google Scholar] [CrossRef]
- Han, G.S.; Kim, S.R. Effects of Endurance Exercise on Mitochondrial Function in Mice. J. Phys. Ther. Sci. 2013, 25, 1317–1319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lundby, C.; Jacobs, R.A. Adaptations of Skeletal Muscle Mitochondria to Exercise Training. Exp. Physiol. 2016, 101, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Çoban, J.; Öztezcan, S.; Doğru-Abbasoğlu, S.; Bingül, I.; Yeşil-Mizrak, K.; Uysal, M. Olive Leaf Extract Decreases Age-Induced Oxidative Stress in Major Organs of Aged Rats. Geriatr. Gerontol. Int. 2014, 14, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, T.; Aloysius Thomas, P.; Geraldine, P. Protective Effect of an Extract of the Oyster Mushroom, Pleurotus Ostreatus, on Antioxidants of Major Organs of Aged Rats. Exp. Gerontol. 2007, 42, 183–191. [Google Scholar] [CrossRef]
- Meng, Q.; Wong, Y.T.; Chen, J.; Ruan, R. Age-Related Changes in Mitochondrial Function and Antioxidative Enzyme Activity in Fischer 344 Rats. Mech. Ageing Dev. 2007, 128, 286–292. [Google Scholar] [CrossRef]
- Finaud, J.; Lac, G.; Filaire, E. Oxidative Stress: Relationship with Exercise and Training. Sport. Med. 2006, 36, 327–358. [Google Scholar] [CrossRef]
- Calbet, J.A.L.; Martín-Rodríguez, S.; Martin-Rincon, M.; Morales-Alamo, D. An Integrative Approach to the Regulation of Mitochondrial Respiration during Exercise: Focus on High-Intensity Exercise. Redox Biol. 2020, 35, 101478. [Google Scholar] [CrossRef]
- Mallikarjuna, K.; Nishanth, K.; Hou, C.W.; Kuo, C.H.; Sathyavelu Reddy, K. Effect of Exercise Training on Ethanol-Induced Oxidative Damage in Aged Rats. Alcohol 2009, 43, 59–64. [Google Scholar] [CrossRef]
- Yoon, G.A.; Park, S. Antioxidant Action of Soy Isoflavones on Oxidative Stress and Antioxidant Enzyme Activities in Exercised Rats. Nutr. Res. Pract. 2014, 8, 618–624. [Google Scholar] [CrossRef] [Green Version]
- Tzimou, A.; Benaki, D.; Nikolaidis, S.; Mikros, E.; Taitzoglou, I.; Mougios, V. Effects of Lifelong Exercise and Aging on the Blood Metabolic Fingerprint of Rats. Biogerontology 2020, 21, 577–591. [Google Scholar] [CrossRef]
- Guide for the Care and Use of Laboratory Animals; National Academy Press: Washington, DC, USA, 1996.






| Groups | Initial Number of Animals | Final Number of Animals | PH | Mortality Rate (%) | Initial Weight (g) | Final Weight (g) | PG | Mean Relative Liver Weight (g) |
|---|---|---|---|---|---|---|---|---|
| NEX 24 | 10 | 9 | 0.63 | 10 | 162.7 ± 13.4 | 471.7 ± 27.8 †,& | 0.65 ± 0.03 †,& | 0.024 ± 0.03 |
| EX24 | 10 | 9 | 0.56 | 10 | 166.3 ± 13.2 | 406.3 ± 14.5 *,& | 0.60 ± 0.03 *,&,# | 0.027 ± 0.01 &,# |
| NEX54 | 10 | 10 | 0.63 | 0 | 152.9 ± 13.4 *,†,# | 546.2 ± 13.6 *,†,# | 0.74 ± 0.02 *,†,# | 0.020 ± 0.05 |
| EX54 | 10 | 10 | 0.55 | 0 | 155.6 ± 11.65 | 439.6 ± 29.7 & | 0.65 ± 0.02 †,& | 0.023 ± 0.02 |
| Groups | Initial Food Consumption (g) | Final Food Consumption (g) | Initial Water Consumption (g) | Final Water Consumption (g) |
|---|---|---|---|---|
| NEX 24 | 16.4 ± 0.2 | 17.2 ± 2.8 | 21.1 ± 1.5 | 16.3 ± 2.7 |
| EX24 | 15.5 ± 5.2 | 19.4 ± 0.8 | 20.9 ± 0.4 | 23.8 ± 1.6 &,* |
| NEX54 | 14.9 ± 0.6 | 19.6 ± 2.8 | 19.6 ± 0.0 | 16.1 ± 0.4 |
| EX54 | 15.9 ± 0.9 | 17.7 ± 0.9 | 17.3 ± 1.0 &,†,* | 20.9 ± 1.6 |
| NEX24 | EX24 | NEX54 | EX54 | |
|---|---|---|---|---|
| SOD (U.min−1.mg−1 protein) | 2.29 ± 0.28 | 2.86 ± 0.21 * | 2.46 ± 0.31 † | 2.79 ± 0.14 * |
| CAT (mmol H2O2.min−1.mg−1 protein) | 0.51 ± 0.071 | 0.58 ± 0.075 | 0.25 ± 0.030 *,† | 0.35 ± 0.06 *,†,& |
| GPx (μmol NADPH oxidized.min−1.mg−1 protein) | 429.4 ± 24.0 | 363.7 ± 40.6 * | 381.9 ± 50.9 * | 342.4 ± 26.6 * |
| GR (μmol NADPH oxidized.min−1.mg−1 protein) | 31.44 ± 2.83 | 25.58 ± 1.71 * | 21.89 ± 2.36 *,† | 20.73 ± 2.13 *,† |
| NEX24 | EX24 | NEX54 | EX54 | |
|---|---|---|---|---|
| LPO (μM MDA.mg−1 protein) | ||||
| Total Fraction | 0.053 ± 0.02 | 1.16 ± 0.26 * | 0.61 ± 0.11 *,† | 0.53 ± 0.11 *,† |
| Mitochondrial Fraction | 0.41 ± 0.14 | 1.24 ± 0.26 * | 0.53 ± 0.06 † | 0.33 ± 0.91 † |
| GSH/GSSG | 3.06 ± 0.25 | 2.68 ± 0.16 | 3.67 ± 0.91 | 4.69 ± 1.19 *,† |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, M.G.; Nunes, P.; Oliveira, P.; Ferreira, R.; Fardilha, M.; Moreira-Gonçalves, D.; Duarte, J.A.; Oliveira, M.M.; Peixoto, F. Long-Term Aerobic Training Improves Mitochondrial and Antioxidant Function in the Liver of Wistar Rats Preventing Hepatic Age-Related Function Decline. Biology 2022, 11, 1750. https://doi.org/10.3390/biology11121750
Silva MG, Nunes P, Oliveira P, Ferreira R, Fardilha M, Moreira-Gonçalves D, Duarte JA, Oliveira MM, Peixoto F. Long-Term Aerobic Training Improves Mitochondrial and Antioxidant Function in the Liver of Wistar Rats Preventing Hepatic Age-Related Function Decline. Biology. 2022; 11(12):1750. https://doi.org/10.3390/biology11121750
Chicago/Turabian StyleSilva, Mónica Garcia, Paulo Nunes, Paula Oliveira, Rita Ferreira, Margarida Fardilha, Daniel Moreira-Gonçalves, José Alberto Duarte, Maria Manuel Oliveira, and Francisco Peixoto. 2022. "Long-Term Aerobic Training Improves Mitochondrial and Antioxidant Function in the Liver of Wistar Rats Preventing Hepatic Age-Related Function Decline" Biology 11, no. 12: 1750. https://doi.org/10.3390/biology11121750
APA StyleSilva, M. G., Nunes, P., Oliveira, P., Ferreira, R., Fardilha, M., Moreira-Gonçalves, D., Duarte, J. A., Oliveira, M. M., & Peixoto, F. (2022). Long-Term Aerobic Training Improves Mitochondrial and Antioxidant Function in the Liver of Wistar Rats Preventing Hepatic Age-Related Function Decline. Biology, 11(12), 1750. https://doi.org/10.3390/biology11121750