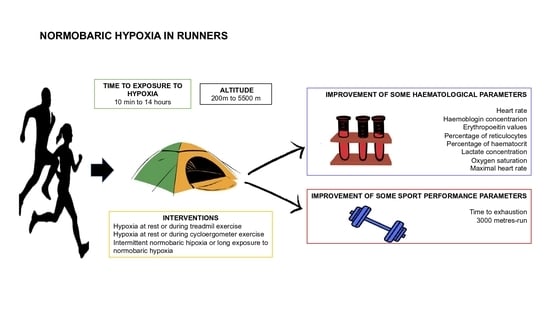
The Effect of Normobaric Hypoxia in Middle- and/or Long-Distance Runners: Systematic Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Objectives
3. Material and Methods
3.1. Elegibility Criteria
3.1.1. Types of Studies
3.1.2. Types of Participants
3.1.3. Language
3.1.4. Publication Date
3.1.5. Exclusion Criteria
3.1.6. Types of Interventions
- -
- Hypoxia at rest or during treadmill exercise
- -
- Hypoxia at rest or during cycloergometer exercise
- -
- Intermittent normobaric hypoxia or long exposure to normobaric hypoxia
3.1.7. Outcome Measure
- •
- Primary
- ○
- Time until exhaustion
- •
- Secondary
- ○
- Haematological parameters
- ○
- Altitude and time under hypoxia
3.2. Data Sources and Search Strategy
3.3. Selection of Studies
3.4. Data Extraction and Management
3.5. Methodological Quality Assessment
4. Results
4.1. Description of the Studies
4.1.1. Search Results
4.1.2. Included Studies
- Study location
- Sample size and years of studies
- Duration of the hypoxia programme
- Participants
- Intervention
Intervention Model
Physical Activity Programme during Exposure to Normobaric Hypoxia
Duration of Exposure to Hypoxia per Session and Duration of the Intervention
Altitude and Hypoxia Simulator Device
4.2. Outcome Measure
4.2.1. Sports Performance Measures
4.2.2. Haematological Parameters
5. Discussion
5.1. Outcome Measures
5.2. Limitations, Perspective and Practical Applications
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McMorris, T.; Hale, B.; Barwood, M.; Costello, J.; Corbett, J. Effect of acute hypoxia on cognition: A systematic review and meta-regression analysis. Neurosci Biobehav. Rev. 2017, 74, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Petrassi, F.A.; Hodkinson, P.D.; Walters, P.L.; Gaydos, S.J. Hypoxic hypoxia at moderate altitudes: Review of the state of the science. Aviat. Space Environ. Med. 2012, 83, 975–984. [Google Scholar] [CrossRef] [PubMed]
- Zoll, J.; Ponsot, E.; Dufour, S.; Doutreleau, S.; Ventura-Clapier, R.; Vogt, M.; Hoppeler, H.; Richard, R.; Flück, M. Exercise training in normobaric hypoxia in endurance runners. III. Muscular adjustments of selected gene transcripts. J. Appl. Physiol. 2006, 100, 1258–1266. [Google Scholar] [CrossRef] [PubMed]
- Vogt, M.; Puntschart, A.; Geiser, J.; Zuleger, C.; Billeter, R.; Hoppeler, H. Molecular adaptations in human skeletal muscle to endurance training under simulated hypoxic conditions. J. Appl. Physiol. 2001, 91, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, A.; Sugita, M.; Kato, K.; Fukuda, A.; Sudo, A.; Uchida, A. Hypoxia Increases Muscle Hypertrophy Induced by Resistance Training. Int. J. Sports Physiol. Perform. 2010, 5, 497–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manimmanakorn, A.; Manimmanakorn, N.; Taylor, R.; Draper, N.; Billaut, F.; Shearman, J.P.; Hamlin, M.J. Effects of resistance training combined with vascular occlusion or hypoxia on neuromuscular function in athletes. Eur. J. Appl. Physiol. 2013, 113, 1767–1774. [Google Scholar] [CrossRef]
- Hopkins, W.G.; Hawley, J.; Burke, L.M. Design and analysis of research on sport performance enhancement. Med. Sci. Sports Exerc. 1999, 31, 472–485. [Google Scholar] [CrossRef]
- Wilber, R.L. Application of altitude/hypoxic training by elite athletes. Med. Sci. Sports Exerc. 2007, 39, 1610–1624. [Google Scholar] [CrossRef] [Green Version]
- Ambroży, T.; Maciejczyk, M.; Klimek, A.T.; Wiecha, S.; Stanula, A.; Snopkowski, P.; Pałka, T.; Jaworski, J.; Ambroży, D.; Rydzik, Ł.; et al. The Effects of Intermittent Hypoxic Training on Anaerobic and Aerobic Power in Boxers. Int. J. Environ. Res. Public Health 2020, 17, 9361. [Google Scholar] [CrossRef]
- Levine, B.D.; Stray-Gundersen, J. Living high-training low: Effect of moderate-altitude acclimatization with low-altitude training on performance. J. Appl. Physiol. 1997, 83, 102–112. [Google Scholar] [CrossRef]
- Levine, B.D. Intermittent Hypoxic Training: Fact and Fancy. High Alt. Med. Biol. 2002, 3, 177–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilber, R.L.; Stray-Gundersen, J.; Levine, B.D. Effect of Hypoxic Dose on Physiological Responses and Sea-Level Performance. Med. Sci. Sports Exerc. 2007, 39, 1590–1599. [Google Scholar] [CrossRef] [PubMed]
- Gore, C.J.; Sharpe, K.; Garvican-Lewis, L.A.; Saunders, P.U.; Humberstone, C.E.; Robertson, E.Y.; Wachsmuth, N.B.; Clark, S.A.; McLean, B.D.; Friedmann-Bette, B.; et al. Altitude training and haemoglobin mass from the optimised carbon monoxide rebreathing method determined by a meta-analysis. Br. J. Sports Med. 2013, 47 (Suppl. 1), i31–i39. [Google Scholar] [CrossRef] [PubMed]
- Scott, B.R.; Slattery, K.M.; Sculley, D.V.; Dascombe, B.J. Hypoxia and Resistance Exercise: A Comparison of Localized and Systemic Methods. Sports Med. 2014, 44, 1037–1054. [Google Scholar] [CrossRef] [PubMed]
- McLean, B.D.; Buttifant, D.; Gore, C.J.; White, K.; Liess, C.; Kemp, J. Physiological and Performance Responses to a Preseason Altitude-Training Camp in Elite Team-Sport Athletes. Int. J. Sports Physiol. Perform. 2013, 8, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Millet, G.P.; Roels, B.; Schmitt, L.; Woorons, X.; Richalet, J.P. Combining Hypoxic Methods for Peak Performance. Sports Med. 2010, 40, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Bonetti, D.L.; Hopkins, W.G. Sea-Level Exercise Performance Following Adaptation to Hypoxia. Sports Med. 2009, 39, 107–127. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro Scale for Rating Quality of Randomized Controlled Trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef] [Green Version]
- Julian, C.G.; Gore, C.J.; Wilber, R.L.; Daniels, J.T.; Fredericson, M.; Stray-Gundersen, J.; Hahn, A.G.; Parisotto, R.; Levine, B.D. Intermittent normobaric hypoxia does not alter performance or erythropoietic markers in highly trained distance runners. J. Appl. Physiol. 2004, 96, 1800–1807. [Google Scholar] [CrossRef] [Green Version]
- Hobbins, L.; Gaoua, N.; Hunter, S.; Girard, O. Psycho-physiological responses to perceptually-regulated interval runs in hypoxia and normoxia. Physiol. Behav. 2019, 209, 112611. [Google Scholar] [CrossRef] [PubMed]
- Brugniaux, J.V.; Schmitt, L.; Robach, P.; Nicolet, G.; Fouillot, J.-P.; Moutereau, S.; Lasne, F.; Pialoux, V.; Saas, P.; Chorvot, M.-C.; et al. Eighteen days of living high, training low stimulate erythropoiesis and enhance aerobic performance in elite middle-distance runners. J. Appl. Physiol. 2006, 100, 203–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uryumtsev, D.Y.; Gultyaeva, V.V.; Zinchenko, M.I.; Baranov, V.I.; Melnikov, V.N.; Balioz, N.V.; Krivoschekov, S.G. Effect of Acute Hypoxia on Cardiorespiratory Coherence in Male Runners. Front. Physiol. 2020, 11, 630. [Google Scholar] [CrossRef] [PubMed]
- Burtscher, M.; Gatterer, H.; Faulhaber, M.; Gerstgrasser, W.; Schenk, K. Effects of Intermittent Hypoxia on Running Economy. Laryngo-Rhino-Otologie 2010, 31, 644–650. [Google Scholar] [CrossRef]
- Dufour, S.P.; Ponsot, E.; Zoll, J.; Doutreleau, S.; Lonsdorfer-Wolf, E.; Geny, B.; Lampert, E.; Flück, M.; Hoppeler, H.; Billat, V.; et al. Exercise training in normobaric hypoxia in endurance runners. I. Improvement in aerobic performance capacity. J. Appl. Physiol. 2006, 100, 1238–1248. [Google Scholar] [CrossRef] [Green Version]
- Hoshikawa, M.; Suzuki, Y.; Oriishi, M. Effects of Normobaric Hypoxia Equivalent to 2,000-m Altitude on Sleep and Physiological Conditions of Athletes. J. Strength Cond. Res. 2013, 27, 2309–2313. [Google Scholar] [CrossRef]
- Katayama, K.; Sato, K.; Matsuo, H.; Ishida, K.; Iwasaki, K.-I.; Miyamura, M. Effect of intermittent hypoxia on oxygen uptake during submaximal exercise in endurance athletes. Graefe’s Arch. Clin. Exp. Ophthalmol. 2004, 92, 75–83. [Google Scholar] [CrossRef]
- Katayama, K.; Sato, K.; Matsuo, H.; Hotta, N.; Sun, Z.; Ishida, K.; Iwasaki, K.-I.; Miyamura, M. Changes in ventilatory responses to hypercapnia and hypoxia after intermittent hypoxia in humans. Respir. Physiol. Neurobiol. 2005, 146, 55–65. [Google Scholar] [CrossRef]
- Neya, M.; Enoki, T.; Kumai, Y.; Sugoh, T.; Kawahara, T. The effects of nightly normobaric hypoxia and high intensity training under intermittent normobaric hypoxia on running economy and hemoglobin mass. J. Appl. Physiol. 2007, 103, 828–834. [Google Scholar] [CrossRef]
- Robertson, E.Y.; Saunders, P.U.; Pyne, D.B.; Gore, C.J.; Anson, J.M. Effectiveness of intermittent training in hypoxia combined with live high/train low. Graefe’s Arch. Clin. Exp. Ophthalmol. 2010, 110, 379–387. [Google Scholar] [CrossRef]
- Robertson, E.Y.; Saunders, P.U.; Pyne, D.B.; Aughey, R.J.; Anson, J.M.; Gore, C.J. Reproducibility of Performance Changes to Simulated Live High/Train Low Altitude. Med. Sci. Sports Exerc. 2010, 42, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Vogt, M.; Hoppeler, H. Is Hypoxia Training Good for Muscles and Exercise Performance? Prog. Cardiovasc. Dis. 2010, 52, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Vallier, J.-M.; Chateau, P.; Guezennec, C.Y. Effects of physical training in a hypobaric chamber on the physical performance of competitive triathletes. Graefe’s Arch. Clin. Exp. Ophthalmol. 1996, 73, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Katayama, K.; Matsuo, H.; Ishida, K.; Mori, S.; Miyamura, M. Intermittent Hypoxia Improves Endurance Performance and Submaximal Exercise Efficiency. High Alt. Med. Biol. 2003, 4, 291–304. [Google Scholar] [CrossRef]
- Sousa, A.C.; Millet, G.P.; Viana, J.; Milheiro, J.; Reis, V. Effects of Normobaric Hypoxia on Matched-severe Exercise and Power-duration Relationship. Laryngo-Rhino-Otologie 2021, 42, 708–715. [Google Scholar] [CrossRef]
- Ashenden, M.; Gore, C.; Dobson, G.; Hahn, A. Live high, train low does not change the total hemoglobin mass of male endurance athletes sleeping at a simulated altitude of 3000m for 23 nights. Eur. J. Appl. Physiol. Occup. Physiol. 1999, 80, 479–484. [Google Scholar] [CrossRef]
- Rusko, H.; Tikkanen, H.; Paavolainen, L.; Hamalainen, I.; Kalliokoski, K.; Puranen, A. Effect of living in hypoxia and training in normoxia on sea level VO2max and red cell mass. Med. Sci. Sports Exerc. 1999, 31, S86. [Google Scholar] [CrossRef]
- Hoshikawa, M.; Uchida, S.; Sugo, T.; Kumai, Y.; Hanai, Y.; Kawahara, T. Changes in sleep quality of athletes under normobaric hypoxia equivalent to 2,000-m altitude: A polysomnographic study. J. Appl. Physiol. 2007, 103, 2005–2011. [Google Scholar] [CrossRef] [Green Version]
- Astorino, T.A.; Allen, R.P.; Roberson, D.W.; Jurancich, M.; Lewis, R.; McCarthy, K.; Trost, E. Adaptations to high-intensity training are independent of gender. Graefe’s Arch. Clin. Exp. Ophthalmol. 2011, 111, 1279–1286. [Google Scholar] [CrossRef]
- Ashenden, M.J.; Gore, C.J.; Martin, D.T.; Dobson, G.P.; Hahn, A.G. Effects of a 12-day “live high, train low” camp on reticulocyte production and haemoglobin mass in elite female road cyclists. Graefe’s Arch. Clin. Exp. Ophthalmol. 1999, 80, 472–478. [Google Scholar] [CrossRef]
- Robach, P.; Schmitt, L.; Brugniaux, J.V.; Nicolet, G.; Duvallet, A.; Fouillot, J.-P.; Moutereau, S.; Lasne, F.; Pialoux, V.; Olsen, N.V.; et al. Living high-training low: Effect on erythropoiesis and maximal aerobic performance in elite Nordic skiers. Eur. J. Appl. Physiol. 2006, 97, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Saunders, P.U.; Telford, R.D.; Pyne, D.B.; Cunningham, R.B.; Gore, C.J.; Hahn, A.G.; Hawley, J.A. Improved running economy in elite runners after 20 days of simulated moderate-altitude exposure. J. Appl. Physiol. 2004, 96, 931–937. [Google Scholar] [CrossRef] [PubMed]

| Name, Year | Scores for Each of the Items | Score | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EC | RA | CA | BC | BS | BT | BA | AF | ITTA | BGC | PEAV | ||
| Brugniaux et al. [22] | No | No | No | No | No | No | No | Yes | Yes | Yes | No | 3/10 |
| Burtscher et al. [24] | No | Yes | No | Yes | No | No | No | Yes | Yes | Yes | No | 5/10 |
| Julian et al. [20] | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | 7/10 |
| Dufour et al. [25] | No | Yes | No | Yes | No | No | No | Yes | Yes | Yes | Yes | 6/10 |
| Hobbins et al. [21] | No | Yes | No | Yes | Yes | No | No | Yes | Yes | Yes | Yes | 7/10 |
| Hoshiwaka et al. [26] | No | No | No | No | No | No | No | Yes | Yes | Yes | Yes | 4/10 |
| Katayama et al. [27] | No | Yes | No | Yes | No | No | No | Yes | Yes | Yes | No | 5/10 |
| Katayama et al. [28] | No | No | No | Yes | No | No | No | Yes | Yes | Yes | No | 4/10 |
| Neya et al. [29] | No | Yes | No | Yes | No | No | No | Yes | Yes | Yes | No | 5/10 |
| Robertson et al. [30] | No | Yes | No | Yes | No | No | No | Yes | Yes | Yes | No | 5/10 |
| Robertson et al. [31] | No | No | No | No | No | No | No | Yes | Yes | Yes | Yes | 4/10 |
| Uryumtsev et al. [23] | No | No | No | No | No | No | No | Yes | Yes | Yes | No | 3/10 |
| Name, Year | n | Sex | Age | VO2 Max mL/kg/min | Duration | Session [Day/Week] | Intervention Moment | Sport | Sport Level |
|---|---|---|---|---|---|---|---|---|---|
| Brugniaux et al., 2006 [22] | 11 | M | 23.5 | 63.3 | 18 days | 7 days/week | - | Middle-distance runners | Elite |
| Butscher et al., 2010 [24] | 11 | M F | 21.8 | 59.65 | 13 weeks | 3 days/week | 2 months after the end of the season | Middle-distance runners | National level |
| Julian et al., 2004 [20] | 14 | F M | 25 | 4.9625 mL/min | 4 weeks | 5 days/week | - | Middle-and-long-distance runners | National level |
| Dufour et al., 2006 [25] | 18 | M | 30.3 | 62.85 | 6 weeks | 2 days/week | - | Long-distance runners | Local athletic teams |
| Hobbins et al., 2019 [21] | 19 | M F | 33.4 | - | 1 week | 3 days/week | - | - | - |
| Hoshiwaka et al., 2013 [26] | 7 | F | 19.6 | - | 1 week | 7 days/week | During the season | Middle-distance runners | Intercollege level |
| Katayama et al., 2004 [27] | 15 | M | 22.2 | - | 2 weeks | 7 days/week | 7 weeks before the championship. | Long-distance runners | Intercollege level |
| Katayama et al., 2004 [28] | 29 | M | 21.05 | - | CONT1:1 week INT2: 2 weeks CONT2:2 weeks | 7 days/week | - | Endurance runners | Intercollege level |
| Neya et al., 2007 [29] | 25 | M | 20.6 | 60.3 | 31 days | INT1: 7 days/week INT2:7 days/week | - | Middle-and-long-distance runners | Intercollege level |
| Robertson et al., 2010 [30] | 17 | M F | - | 65.5 | 3 weeks | 4 days/week | Middle-distance runners | - | |
| Robertson et al., 2010 [31] | 16 | M F | - | 68.75 | 6 weeks | 7 days/week | Middle-and-long-distance runners | Elite | |
| Uryumtsev et al., 2020 [23] | 20 | M | 21.5 | - | - | - | - | Middle-distance runners | - |
| Name, Year | Exposure Type | Exposure Time | Altitude Simulated/Saturation | Administration Hypoxia | Hypoxia Moment | Other |
|---|---|---|---|---|---|---|
| Brugniaux et al., 2006 [22] | INT1: HR (sleeping) CONT1: no hypoxia | INT1: 14 h/day | HR: (FiO2 = 6 nights at 2500 m/0.174, 12 nights at 3000 m/0.164). CONT1:1200 m | Hypoxic room | - | INT1 and CONT trained at 1200 m normoxia |
| Butscher et al., 2010 [24] | INT1: HR (position not described) CONT1: no hypoxia | 2 h session/3 times per week for 10 weeks | FiO2 = 15%(3200 m)–11% (5500 m) | Hypoxic room | - | - |
| Julian et al., 2004 [20] | INT1: HR CONT1: no hypoxia | 5:5 min during 70 min, 5 times a week. | INT1: FiO2 changed from week 1 to week 4, was: 0.12, 0.11, 0.10, 0.10, respectively. CONT1: FiO2 = 0.209 | Hypoxic room | 1–2 h after or before exercise training | - |
| Dufour et al., 2006 [25] | INT1: HE. Different each week; 24 to 40 min in treadmill CONT: HE | Week 3 and 6: 24 min/session Week 4 and 7: 32 min/session Week 5 and 8: 40 min/session | INT1: (FiO2 = 14.5%) o 3000 m CONT1: (FiO2 = 20.9%) | Facemask | - | - |
| Hobbins et al., 2019 [21] | INT1: HE (4 × 4 min running × 3 min at rest (28 min total hypoxia) CONT1: no hypoxia | 28 min/session. (2 sesions) | INT1: (FiO2 = 15% o 2700 m) CONT1:(FiO2 = 20.9%) | Facemask | - | - |
| Hoshiwaka et al., 2013 [26] | INT1: HR (sleeping) and HE (cyclo-ergometer and treadmill) | HR: 7 h/night (6 nights) HE: 1 h aprox/session | HR: (FiO2 = 16.4% or 2000 m) HE: (FiO2 = 14.4% or 3000 m) | HR: hypoxic room HE: (not indicated) | - | - |
| Katayama et al., 2004 [27] | INT1: HR CONT1: no hypoxia | 3 h/session during 14 consecutive days. | INT1: (FiO2 = 12.3%) CONT1: no hypoxia | Hypoxic tent | - | |
| Katayama et al., 2004 [28] | INT1 and CONT1:HR (sitting) INT2 and CONT2: HR (sitting) | INT1 and CONT1: 3 h/day during 1 week INT2 and CONT2: 3 h/day during 2 weeks | INT1 and INT2: FiO2= 12.3–12% CONT1 and CONT2= normal FiO2 (no hypoxia) | Hypoxic tent | - | - |
| Neya et al., 2007 [29] | INT1: HR (sleeping) INT2: HE 30 min treadmill CONT1: HE | INT1: 10–12 h during 29 nights. INT2: 30 min during 12 days CONT1: no hypoxia | INT1:3000 m (FiO2 = 0.144) INT2:3000 m (FiO2 = 0.144) | Hypoxic room INT1: 50 m3 INT2: 100 m3 | - | - |
| Robertson et al., 2010 [30] | INT1: HE (treadmill) INT2: HR y HE (treadmill) | INT1: 4–5 h hypoxia in exercise/week INT2: 4–5 h hypoxia in exercise/week +14 h per day 3000 m rest | HE: 2200 m HR: 3000 m | Hypoxic room | - | - |
| Robertson et al., 2009 [31] | INT1: HR (Not described) CONT1: no hypoxia | INT1:14 h/day | INT1:(FiO2 = 3000 m) CONT1:resided near sea level (600 m) | Hypoxic room | - | |
| Uryumtsev et al., 2020 [23] | INT1: HR (sitting) | 10:10 min | (FiO2 = 10%) | Facemask | - | - |
| Name, Year | Maximal Heart Rate or Heart Rate | Hemoglobin Concentration | Percentage of Hematocrit | Lactate Concentration | Percentage of Reticulocytes | Erythropoietin Values | Oxygen Saturation |
|---|---|---|---|---|---|---|---|
| Brugniaux et al., 2006 [22] | HRmax: No significant difference between the groups. | ||||||
| Butscher et al., 2010 [24] | Significantly increased during the 5th week in comparison to the pre-intervention data, but this improvement did not remain during weeks 8 and 13. There were no significant differences between the groups (p > 0.05) | Significant increase in the intervention group during the 5 weeks of training in comparison with the values obtained before starting the intervention. However, there were no significant changes when comparing the two following measures during weeks 8 and 13 with the values from pre-intervention (p > 0.05). | |||||
| Julian et al., 2004 [20] | No significant difference between the groups | No significant differences between the values obtained after the intervention and those obtained pre-intervention (p > 0.05) | No significant changes (p > 0.05) | Significant increase in both groups after 12 days of intervention in comparison to 10 days after finishing (p < 0.05) | The group exposed to hypoxia obtained a significant decrease in comparison to the value before starting (p < 0.05) | ||
| Dufour et al., 2006 [25] | HR max: no significant difference between the groups | No significant changes (p > 0.05) | |||||
| Hobbins et al., 2019 [21] | Major increase in INT1 (p < 0.05). | Significantly smaller (p < 0.01) in INT1 group in comparison to the CONT1 group. | |||||
| Hoshiwaka et al., 2013 [26] | HR: values on the 1st night under hypoxia compared with those under normoxia indicated a significant increase after hypoxia (p < 0.05). | Decreased on the first night sleeping in oxygen-poor air conditions and on the sixth night (the last one of intervention), in comparison to the night in normoxia (p < 0.05). | |||||
| Katayama et al., 2004 [27] | HR max: no differences between the groups (p > 0.05) HR: decreased (p < 0.05) after IH. Decreased (first to final night under hypoxia) (p < 0.05) | Any significant rise was produced in that measure after the intervention. No significant differences between groups (p > 0.05) | No significant difference in this outcome measure for the INT1 group after the hypoxia, and no significant differences between the groups (p < 0.05) | No significant improvements in favour of INT1 group, and no significant improvements found when comparing this group with the CONT1 group (p < 0.05). | No significant changes in the INT1 group after the exposure and no significant changes between the groups (p < 0.05) | ||
| Katayama et al., 2004 [28] | INT2 values increased after the end of the intervention (p < 0.05). However, after 2 weeks the values were the same as the initial values. INT1 increased significantly after the end of the intervention (p < 0.05) | ||||||
| Neya et al., 2007 [29] | HR: no significant differences in the INT1 and INT2 after being exposed to hypoxia (p < 0.05) | No significant changes (p > 0.05) | |||||
| Robertson et al., 2010 [30] | Compared with INT1, INT2 had substantially higher values at week 1, 2 and 3. | No significant changes (p > 0.05) | INT2 substantially increased during weeks 1 and 3. No data for week 2. INT1 had no substantial changes in percent reticulocytes (p > 0.05). | INT1 had no substantial changes in percent reticulocytes. | |||
| Robertson et al., 2010 [31] | INT1 had substantially higher values after block 2 than CONT1. After block 1 the differences were negligible. | Significant increase in INT1 during days 2 and 6 of exposure, both in block 1 and 2. Even so, these effects did not remain during days 20 and 27 in either of the two blocks of the intervention. | |||||
| Uryumtsev et al., 2020 [23] | HR: NT1 increased significantly (p < 0.05) during hypoxia by 31%. | INT1 decreased (p < 0.05) during hypoxia by 21% |
| Name, Year | 3000 m-Run: | Time to Exhaustion |
|---|---|---|
| Julian et al., 2004 [20] | Did not improve after hypoxia exposure (p > 0.05) | |
| Dufour et al., 2006 [25] | Significantly improved (p < 0.05) | |
| Katayama et al., 2004 [27] | Improving trend after intervention (p = 0.06) | Significantly improved (p < 0.05) |
| Katayama et al., 2004 [28] | ||
| Neya et al., 2007 [29] | The groups exposed to hypoxia trend to significance (p = 0.07) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albertus-Cámara, I.; Ferrer-López, V.; Martínez-González-Moro, I. The Effect of Normobaric Hypoxia in Middle- and/or Long-Distance Runners: Systematic Review. Biology 2022, 11, 689. https://doi.org/10.3390/biology11050689
Albertus-Cámara I, Ferrer-López V, Martínez-González-Moro I. The Effect of Normobaric Hypoxia in Middle- and/or Long-Distance Runners: Systematic Review. Biology. 2022; 11(5):689. https://doi.org/10.3390/biology11050689
Chicago/Turabian StyleAlbertus-Cámara, Inés, Vicente Ferrer-López, and Ignacio Martínez-González-Moro. 2022. "The Effect of Normobaric Hypoxia in Middle- and/or Long-Distance Runners: Systematic Review" Biology 11, no. 5: 689. https://doi.org/10.3390/biology11050689
APA StyleAlbertus-Cámara, I., Ferrer-López, V., & Martínez-González-Moro, I. (2022). The Effect of Normobaric Hypoxia in Middle- and/or Long-Distance Runners: Systematic Review. Biology, 11(5), 689. https://doi.org/10.3390/biology11050689