Both Nuclear and Membrane Estrogen Receptor Alpha Impact the Expression of Estrogen Receptors and Plasticity Markers in the Mouse Hypothalamus and Hippocampus
Abstract
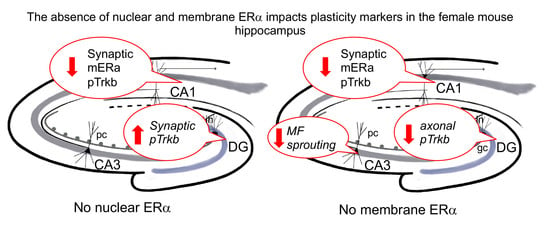
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Tissue Preparation
2.3. Antibodies
2.4. Light Microscopic Immunocytochemistry
2.5. Electron Microscopic Immunocytochemistry
2.6. Figure Preparation
3. Results
3.1. Examination of Nuclear ERα Labeling in the Hypothalamus
3.2. Examination of ERβ Labeling in the Hypothalamus
3.3. Examination of Extranuclear ERα Labeling in the Hippocampus
3.4. Examination of pTrKb Labeling in the Hippocampus
3.5. Examination of Dyn and ZnT3 in the Mossy Fiber Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABC | avidin–biotin complex |
| ANOVA | analysis of variance |
| BSA | bovine serum albumin |
| DAB | diaminobenzidine |
| DG | dentate gyrus |
| Dyn | dynorphin |
| EM | immunoelectron microscopy |
| ER | estrogen receptor |
| ES | embryonic stem |
| Ir | immunoreactivity |
| KI | knock-in |
| MOER | membrane only estrogen receptor alpha |
| NOER | nuclear only estrogen receptor alpha |
| PB | phosphate buffer |
| PFA | paraformaldehyde |
| PVN | paraventricular nucleus |
| ROI | region of interest |
| SLu | stratum lucidum |
| SO | stratum oriens |
| TS | tris-buffered saline |
| WT | wildtype |
References
- Arnal, J.F.; Lenfant, F.; Metivier, R.; Flouriot, G.; Henrion, D.; Adlanmerini, M.; Fontaine, C.; Gourdy, P.; Chambon, P.; Katzenellenbogen, B.; et al. Membrane and Nuclear Estrogen Receptor Alpha Actions: From Tissue Specificity to Medical Implications. Physiol. Rev. 2017, 97, 1045–1087. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.R. Integration of the Extranuclear and Nuclear Actions of Estrogen. Mol. Endocrinol. 2005, 19, 1951–1959. [Google Scholar] [CrossRef]
- Razandi, M.; Pedram, A.; Merchenthaler, I.; Greene, G.L.; Levin, E.R. Plasma Membrane Estrogen Receptors Exist and Functions as Dimers. Mol. Endocrinol. 2004, 18, 2854–2865. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, N.; Pfaff, D.W. Non-genomic actions of estrogens and their interaction with genomic actions in the brain. Front. Neuroendocr. 2008, 29, 238–257. [Google Scholar] [CrossRef]
- Adlanmerini, M.; Solinhac, R.; Abot, A.; Fabre, A.; Raymond-Letron, I.; Guihot, A.-L.; Boudou, F.; Sautier, L.; Vessières, E.; Kim, S.H.; et al. Mutation of the palmitoylation site of estrogen receptor α in vivo reveals tissue-specific roles for membrane versus nuclear actions. Proc. Natl. Acad. Sci. USA 2014, 111, E283–E290. [Google Scholar] [CrossRef]
- McEwen, B.S.; Milner, T.A. Understanding the broad influence of sex hormones and sex differences in the brain. J. Neurosci. Res. 2017, 95, 24–39. [Google Scholar] [CrossRef]
- Spencer-Segal, J.; Tsuda, M.; Mattei, L.; Waters, E.; Romeo, R.; Milner, T.; McEwen, B.; Ogawa, S. Estradiol acts via estrogen receptors alpha and beta on pathways important for synaptic plasticity in the mouse hippocampal formation. Neuroscience 2012, 202, 131–146. [Google Scholar] [CrossRef]
- Contoreggi, N.H.; Mazid, S.; Goldstein, L.B.; Park, J.; Ovalles, A.C.; Waters, E.M.; Glass, M.J.; Milner, T.A. Sex and age influence gonadal steroid hormone receptor distributions relative to estrogen receptor β-containing neurons in the mouse hypothalamic paraventricular nucleus. J. Comp. Neurol. 2021, 529, 2283–2310. [Google Scholar] [CrossRef]
- Mitterling, K.L.; Spencer-Segal, J.; Dziedzic, N.; Shenoy, S.; McCarthy, K.; Waters, E.M.; McEwen, B.S.; Milner, T.A. Cellular and subcellular localization of estrogen and progestin receptor immunoreactivities in the mouse hippocampus. J. Comp. Neurol. 2010, 518, 2729–2743. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S.; Milner, T.A. Hippocampal formation: Shedding light on the influence of sex and stress on the brain. Brain Res. Rev. 2007, 55, 343–355. [Google Scholar] [CrossRef]
- Spencer, J.L.; Waters, E.M.; Romeo, R.D.; Wood, G.E.; Milner, T.A.; McEwen, B.S. Uncovering the mechanisms of estrogen effects on hippocampal function. Front. Neuroendocr. 2008, 29, 219–237. [Google Scholar] [CrossRef] [PubMed]
- Milner, T.A.; Contoreggi, N.H.; Yu, F.; Johnson, M.A.; Wang, G.; Woods, C.; Mazid, S.; Van Kempen, T.A.; Waters, E.M.; McEwen, B.S.; et al. Estrogen Receptor β Contributes to Both Hypertension and Hypothalamic Plasticity in a Mouse Model of Peri-Menopause. J. Neurosci. 2021, 41, 5190–5205. [Google Scholar] [CrossRef] [PubMed]
- Rune, G.; Wehrenberg, U.; Prange-Kiel, J.; Zhou, L.; Adelmann, G.; Frotscher, M. Estrogen up-regulates estrogen receptor α and synaptophysin in slice cultures of rat hippocampus. Neuroscience 2002, 113, 167–175. [Google Scholar] [CrossRef]
- Spencer-Segal, J.L.; Waters, E.M.; Bath, K.G.; Chao, M.V.; McEwen, B.S.; Milner, T.A. Distribution of Phosphorylated TrkB Receptor in the Mouse Hippocampal Formation Depends on Sex and Estrous Cycle Stage. J. Neurosci. 2011, 31, 6780–6790. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.; Milner, T.; McEwen, B. Dynamic plasticity: The role of glucocorticoids, brain-derived neurotrophic factor and other trophic factors. Neuroscience 2013, 239, 214–227. [Google Scholar] [CrossRef]
- Scharfman, H.E.; MacLusky, N.J. Sex differences in the neurobiology of epilepsy: A preclinical perspective. Neurobiol. Dis. 2014, 72 Pt B, 180–192. [Google Scholar] [CrossRef]
- Torres-Reveron, A.; Khalid, S.; Williams, T.; Waters, E.; Jacome, L.; Luine, V.; Drake, C.; McEwen, B.; Milner, T. Hippocampal dynorphin immunoreactivity increases in response to gonadal steroids and is positioned for direct modulation by ovarian steroid receptors. Neuroscience 2009, 159, 204–216. [Google Scholar] [CrossRef]
- Van Kempen, T.A.; Kahlid, S.; Gonzalez, A.D.; Spencer-Segal, J.L.; Tsuda, M.C.; Ogawa, S.; McEwen, B.S.; Waters, E.M.; Milner, T.A. Sex and estrogen receptor expression influence opioid peptide levels in the mouse hippocampal mossy fiber pathway. Neurosci. Lett. 2013, 552, 66–70. [Google Scholar] [CrossRef]
- Levin, E.R. Membrane estrogen receptors signal to determine transcription factor function. Steroids 2018, 132, 1–4. [Google Scholar] [CrossRef]
- Pedram, A.; Razandi, M.; Lewis, M.; Hammes, S.; Levin, E.R. Membrane-Localized Estrogen Receptor α Is Required for Normal Organ Development and Function. Dev. Cell 2014, 29, 482–490. [Google Scholar] [CrossRef]
- Pedram, A.; Razandi, M.; Kim, J.K.; O’Mahony, F.; Lee, E.Y.; Luderer, U.; Levin, E.R. Developmental Phenotype of a Membrane Only Estrogen Receptor α (MOER) Mouse. J. Biol. Chem. 2009, 284, 3488–3495. [Google Scholar] [CrossRef] [PubMed]
- Milner, T.A.; Waters, E.M.; Robinson, D.C.; Pierce, J.P. Degenerating Processes Identified by Electron Microscopic Immunocytochemical Methods. Neurodegener. Methods Protoc. 2011, 793, 23–59. [Google Scholar] [CrossRef]
- Svingos, A.L.; Colago, E.E.; Pickel, V.M. Cellular sites for dynorphin activation of kappa-opioid receptors in the rat nucleus accumbens shell. J. Neurosci. 1999, 19, 1804–1813. [Google Scholar] [CrossRef]
- Okamura, H.; Yamamoto, K.; Hayashi, S.; Kuroiwa, A.; Muramatsu, M. A polyclonal antibody to the rat oestrogen receptor expressed in Escherichia coli: Characterization and application to immunohistochemistry. J. Endocrinol. 1992, 135, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Alves, E.S.; Weiland, N.G.; Hayashi, S.; McEwen, B.S. Immunocytochemical localization of nuclear estrogen receptors and progestin receptors within the rat dorsal raphe nucleus. J. Comp. Neurol. 1998, 391, 322–334. [Google Scholar] [CrossRef]
- Milner, A.T.; McEwen, B.S.; Hayashi, S.; Li, C.J.; Reagan, L.P.; Alves, E.S. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. J. Comp. Neurol. 2001, 429, 355–371. [Google Scholar] [CrossRef]
- Shughrue, P.J.; Merchenthaler, I. Distribution of estrogen receptor beta immunoreactivity in the rat central nervous system. J. Comp. Neurol. 2001, 436, 64–81. [Google Scholar] [CrossRef]
- Bath, K.G.; Mandairon, N.; Jing, D.; Rajagopal, R.; Kapoor, R.; Chen, Z.-Y.; Khan, T.; Proenca, C.C.; Kraemer, R.; Cleland, T.A.; et al. Variant Brain-Derived Neurotrophic Factor (Val66Met) Alters Adult Olfactory Bulb Neurogenesis and Spontaneous Olfactory Discrimination. J. Neurosci. 2008, 28, 2383–2393. [Google Scholar] [CrossRef]
- Alderson, R.F.; Curtis, R.; Alterman, A.L.; Lindsay, R.M.; DiStefano, P.S. Truncated TrkB mediates the endocytosis and release of BDNF and neurotrophin-4/5 by rat astrocytes and Schwann cells in vitro. Brain Res. 2000, 871, 210–222. [Google Scholar] [CrossRef]
- Rose, C.R.; Blum, R.; Pichler, B.; Lepier, A.; Kafitz, K.W.; Konnerth, A. Truncated TrkB-T1 mediates neurotrophin-evoked calcium signalling in glia cells. Nature 2003, 426, 74–78. [Google Scholar] [CrossRef]
- Ohira, K.; Kumanogoh, H.; Sahara, Y.; Homma, K.J.; Hirai, H.; Nakamura, S.; Hayashi, M. A Truncated Tropo-Myosine-Related Kinase B Receptor, T1, Regulates Glial Cell Morphology via Rho GDP Dissociation Inhibitor. J. Neurosci. 2005, 25, 1343–1353. [Google Scholar] [CrossRef]
- Ohira, K.; Funatsu, N.; Homma, K.J.; Sahara, Y.; Hayashi, M.; Kaneko, T.; Nakamura, S. Truncated TrkB-T1 regulates the morphology of neocortical layer I astrocytes in adult rat brain slices. Eur. J. Neurosci. 2007, 25, 406–416. [Google Scholar] [CrossRef]
- Carim-Todd, L.; Bath, K.G.; Fulgenzi, G.; Yanpallewar, S.; Jing, D.; Barrick, C.A.; Becker, J.; Buckley, H.; Dorsey, S.G.; Lee, F.S.; et al. Endogenous Truncated TrkB.T1 Receptor Regulates Neuronal Complexity and TrkB Kinase Receptor Function In Vivo. J. Neurosci. 2009, 29, 678–685. [Google Scholar] [CrossRef]
- Wenzel, H.J.; Cole, T.B.; Born, D.E.; Schwartzkroin, P.A.; Palmiter, R.D. Ultrastructural localization of zinc transporter-3 (ZnT-3) to synaptic vesicle membranes within mossy fiber boutons in the hippocampus of mouse and monkey. Proc. Natl. Acad. Sci. USA 1997, 94, 12676–12681. [Google Scholar] [CrossRef] [PubMed]
- Auchus, A.P.; Pickel, V.M. Quantitative light microscopic demonstration of increased pallidal and striatal met5-enkephalin-like immunoreactivity in rats following chronic treatment with haloperidol but not with clozapine: Implications for the pathogenesis of neuroleptic-induced movement disorders. Exp. Neurol. 1992, 117, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Pierce, J.P.; Kelter, D.T.; McEwen, B.S.; Waters, E.M.; Milner, T.A. Hippocampal mossy fiber leu-enkephalin immunoreactivity in female rats is significantly altered following both acute and chronic stress. J. Chem. Neuroanat. 2014, 55, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.; Palay, S.; Webster, H. Fine Structure of the Nervous System: Neurons and Their Supporting Cells, 3rd ed.; Oxford University Press: Oxford, UK, 1991. [Google Scholar]
- Razandi, M.; Pedram, A.; Greene, G.L.; Levin, E.R. Cell Membrane and Nuclear Estrogen Receptors (ERs) Originate from a Single Transcript: Studies of ERα and ERβ Expressed in Chinese Hamster Ovary Cells. Mol. Endocrinol. 1999, 13, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Micevych, P.; Dominguez, R. Membrane estradiol signaling in the brain. Front. Neuroendocr. 2009, 30, 315–327. [Google Scholar] [CrossRef]
- Pierce, J.P.; Melton, J.; Punsoni, M.; McCloskey, D.P.; Scharfman, H.E. Mossy fibers are the primary source of afferent input to ectopic granule cells that are born after pilocarpine-induced seizures. Exp. Neurol. 2005, 196, 316–331. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Kim, J.-H.; Hong, S.H.; Lee, J.Y.; Cherny, R.A.; Bush, A.I.; Palmiter, R.D.; Koh, J.-Y. Estrogen Decreases Zinc Transporter 3 Expression and Synaptic Vesicle Zinc Levels in Mouse Brain. J. Biol. Chem. 2004, 279, 8602–8607. [Google Scholar] [CrossRef]
- Xue, B.; Zhang, Z.; Beltz, T.G.; Johnson, R.F.; Guo, F.; Hay, M.; Johnson, A.K. Estrogen Receptor-β in the Paraventricular Nucleus and Rostroventrolateral Medulla Plays an Essential Protective Role in Aldosterone/Salt-Induced Hypertension in Female Rats. Hypertension 2013, 61, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Weiland, N.; Orikasa, C.; Hayashi, S.; McEwen, B. Distribution and hormone regulation of estrogen receptor immunoreactive cells in the hippocampus of male and female rats. J. Comp. Neurol. 1997, 388, 603–612. [Google Scholar] [CrossRef]
- Isgor, C.; Watson, S. Estrogen receptor α and β mRNA expressions by proliferating and differentiating cells in the adult rat dentate gyrus and subventricular zone. Neuroscience 2005, 134, 847–856. [Google Scholar] [CrossRef]
- Tanapat, P.; Hastings, N.B.; Reeves, A.J.; Gould, E. Estrogen Stimulates a Transient Increase in the Number of New Neurons in the Dentate Gyrus of the Adult Female Rat. J. Neurosci. 1999, 19, 5792–5801. [Google Scholar] [CrossRef]
- Sheppard, P.A.S.; Choleris, E.; Galea, L.A.M. Structural plasticity of the hippocampus in response to estrogens in female rodents. Mol. Brain 2019, 12, 22. [Google Scholar] [CrossRef] [PubMed]
- Hart, S.A.; Patton, J.D.; Woolley, C.S. Quantitative analysis of ER? and GAD colocalization in the hippocampus of the adult female rat. J. Comp. Neurol. 2001, 440, 144–155. [Google Scholar] [CrossRef]
- Waters, E.M.; Thompson, L.I.; Patel, P.; Gonzales, A.D.; Ye, H.; Filardo, E.J.; Clegg, D.J.; Gorecka, J.; Akama, K.T.; McEwen, B.S.; et al. G-Protein-Coupled Estrogen Receptor 1 Is Anatomically Positioned to Modulate Synaptic Plasticity in the Mouse Hippocampus. J. Neurosci. 2015, 35, 2384–2397. [Google Scholar] [CrossRef]
- Xu, W.; Cao, J.; Zhou, Y.; Wang, L.; Zhu, G. GPR30 activation improves memory and facilitates DHPG-induced LTD in the hippocampal CA3 of middle-aged mice. Neurobiol. Learn. Mem. 2018, 149, 10–19. [Google Scholar] [CrossRef]
- Briz, V.; Liu, Y.; Zhu, G.; Bi, X.; Baudry, M. A novel form of synaptic plasticity in field CA3 of hippocampus requires GPER1 activation and BDNF release. J. Cell Biol. 2015, 210, 1225–1237. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazid, S.; Waters, E.M.; Lopez-Lee, C.; Poultan Kamakura, R.; Rubin, B.R.; Levin, E.R.; McEwen, B.S.; Milner, T.A. Both Nuclear and Membrane Estrogen Receptor Alpha Impact the Expression of Estrogen Receptors and Plasticity Markers in the Mouse Hypothalamus and Hippocampus. Biology 2023, 12, 632. https://doi.org/10.3390/biology12040632
Mazid S, Waters EM, Lopez-Lee C, Poultan Kamakura R, Rubin BR, Levin ER, McEwen BS, Milner TA. Both Nuclear and Membrane Estrogen Receptor Alpha Impact the Expression of Estrogen Receptors and Plasticity Markers in the Mouse Hypothalamus and Hippocampus. Biology. 2023; 12(4):632. https://doi.org/10.3390/biology12040632
Chicago/Turabian StyleMazid, Sanoara, Elizabeth M. Waters, Chloe Lopez-Lee, Renata Poultan Kamakura, Batsheva R. Rubin, Ellis R. Levin, Bruce S. McEwen, and Teresa A. Milner. 2023. "Both Nuclear and Membrane Estrogen Receptor Alpha Impact the Expression of Estrogen Receptors and Plasticity Markers in the Mouse Hypothalamus and Hippocampus" Biology 12, no. 4: 632. https://doi.org/10.3390/biology12040632
APA StyleMazid, S., Waters, E. M., Lopez-Lee, C., Poultan Kamakura, R., Rubin, B. R., Levin, E. R., McEwen, B. S., & Milner, T. A. (2023). Both Nuclear and Membrane Estrogen Receptor Alpha Impact the Expression of Estrogen Receptors and Plasticity Markers in the Mouse Hypothalamus and Hippocampus. Biology, 12(4), 632. https://doi.org/10.3390/biology12040632