Exosomal Non-Coding RNA Mediates Macrophage Polarization: Roles in Cardiovascular Diseases
Abstract
:Simple Summary
Abstract
1. Introduction
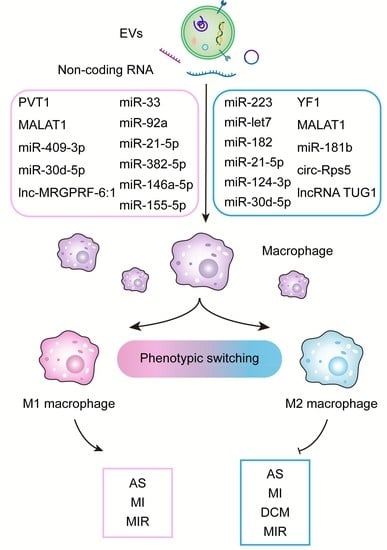
2. Molecular Mechanism of Exosomal ncRNA Regulating Macrophage Polarization
3. Role of Exosomal ncRNA in CVD-Associated Macrophage Polarization
3.1. Atherosclerosis
3.2. Myocardial Infarction and Ischemia-Reperfusion Injury
3.3. Cardiomyopathy
3.4. Diabetic Cardiomyopathy
3.5. Viral Myocarditis
4. Regulation of Macrophage Polarization by Immune Cell-Derived EVs and Their Role in Cardiovascular Disease
5. Role of Polarized Macrophage-Derived EVs in CVD
6. A Role for EVs in the Treatment of CVD by Acting on Macrophages
6.1. Stem Cells-Derived EVs
6.2. Engineered EVs
6.3. Important but Blank Space for Macrophage Regulation in CVD
6.3.1. Plant-Derived Exosome-like Nanovesicles
6.3.2. Microparticles Derived from Erythrocytes and Platelets
7. Limitations
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bodenlos, K.; Brandt, J.S.; Graham, H.L.; Schuster, M.; Ananth, C.V. Trends in cardiovascular disease-related maternal mortality in the United States, 1999–2018. Am. J. Obstet. Gynecol. 2022, 226, 432–434. [Google Scholar] [CrossRef] [PubMed]
- Townsend, N.; Wilson, L.; Bhatnagar, P.; Wickramasinghe, K.; Rayner, M.; Nichols, M. Cardiovascular disease in Europe: Epidemiological update 2016. Eur. Heart J. 2016, 37, 3232–3245. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Rosenzweig, A. Exercise and cardiovascular protection: Update and future. J. Sport Health Sci. 2021, 10, 607–608. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Liu, J.; Wang, M.; Zhang, X.; Zhou, M. Epidemiology of cardiovascular disease in China: Current features and implications. Nat. Rev. Cardiol. 2019, 16, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Bei, Y.; Wang, L.; Ding, R.; Che, L.; Fan, Z.; Gao, W.; Liang, Q.; Lin, S.; Liu, S.; Lu, X.; et al. Animal exercise studies in cardiovascular research: Current knowledge and optimal design-A position paper of the Committee on Cardiac Rehabilitation, Chinese Medical Doctors’ Association. J. Sport Health Sci. 2021, 10, 660–674. [Google Scholar] [CrossRef]
- Wang, H.; Maimaitiaili, R.; Yao, J.; Xie, Y.; Qiang, S.; Hu, F.; Li, X.; Shi, C.; Jia, P.; Yang, H.; et al. Percutaneous Intracoronary Delivery of Plasma Extracellular Vesicles Protects the Myocardium Against Ischemia-Reperfusion Injury in Canis. Hypertension 2021, 78, 1541–1554. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xie, Y.; Salvador, A.M.; Zhang, Z.; Chen, K.; Li, G.; Xiao, J. Exosomes: Multifaceted Messengers in Atherosclerosis. Curr. Atheroscler. Rep. 2020, 22, 57. [Google Scholar] [CrossRef]
- Lou, J.; Wu, J.; Feng, M.; Dang, X.; Wu, G.; Yang, H.; Wang, Y.; Li, J.; Zhao, Y.; Shi, C.; et al. Exercise promotes angiogenesis by enhancing endothelial cell fatty acid utilization via liver-derived extracellular vesicle miR-122-5p. J. Sport Health Sci. 2022, 11, 495–508. [Google Scholar] [CrossRef]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Cheng, L.; Hill, A.F. Therapeutically harnessing extracellular vesicles. Nat. Rev. Drug Discov. 2022, 21, 379–399. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Munoz, E.R.; Caccese, J.B.; Wilson, B.E.; Shuler, K.T.; Santos, F.V.; Caban, C.T.; Jeka, J.J.; Langford, D.; Hudson, M.B. Effects of purposeful soccer heading on circulating small extracellular vesicle concentration and cargo. J. Sport Health Sci. 2021, 10, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef]
- Li, C.; Ni, Y.Q.; Xu, H.; Xiang, Q.Y.; Zhao, Y.; Zhan, J.K.; He, J.Y.; Li, S.; Liu, Y.S. Roles and mechanisms of exosomal non-coding RNAs in human health and diseases. Signal Transduct. Target. Ther. 2021, 6, 383. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.P.; Boon, R.A. Exosomes and non-coding RNA, the healers of the heart? Cardiovasc. Res. 2020, 116, 258–259. [Google Scholar] [CrossRef] [PubMed]
- Del Campo, C.V.; Liaw, N.Y.; Gunadasa-Rohling, M.; Matthaei, M.; Braga, L.; Kennedy, T.; Salinas, G.; Voigt, N.; Giacca, M.; Zimmermann, W.H.; et al. Regenerative potential of epicardium-derived extracellular vesicles mediated by conserved miRNA transfer. Cardiovasc. Res. 2022, 118, 597–611. [Google Scholar] [CrossRef] [PubMed]
- Riffo-Campos, A.L.; Perez-Hernandez, J.; Ortega, A.; Martinez-Arroyo, O.; Flores-Chova, A.; Redon, J.; Cortes, R. Exosomal and Plasma Non-Coding RNA Signature Associated with Urinary Albumin Excretion in Hypertension. Int. J. Mol. Sci. 2022, 23, 823. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Natoli, G.; Monticelli, S. Macrophage activation: Glancing into diversity. Immunity 2014, 40, 175–177. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Treiber, T.; Treiber, N.; Meister, G. Regulation of microRNA biogenesis and its crosstalk with other cellular pathways. Nat. Rev. Mol. Cell Biol. 2019, 20, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Iadevaia, V.; Gerber, A.P. Combinatorial Control of mRNA Fates by RNA-Binding Proteins and Non-Coding RNAs. Biomolecules 2015, 5, 2207–2222. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, L.; Liang, X.; Cao, Y.; Zhu, X.; Wang, S.; Li, J.; Gao, J.; Xiao, J. Exercise attenuates angiotensinⅡ-induced muscle atrophy by targeting PPARgamma/miR-29b. J. Sport Health Sci. 2022, 11, 696–707. [Google Scholar] [CrossRef]
- Yang, T.; Ai, S.; Gokulnath, P.; Li, G.; Xiao, J. Cellular and Extracellular Non-coding RNAs in Cardiac Physiology and Diseases. J. Cardiovasc. Transl. Res. 2022, 15, 441–443. [Google Scholar] [CrossRef]
- Wang, H.; Wang, T.; Rui, W.; Xie, J.; Xie, Y.; Zhang, X.; Guan, L.; Li, G.; Lei, Z.; Schiffelers, R.M.; et al. Extracellular vesicles enclosed-miR-421 suppresses air pollution (PM2.5)-induced cardiac dysfunction via ACE2 signalling. J. Extracell. Vesicles 2022, 11, e12222. [Google Scholar] [CrossRef]
- Gou, W.; Zhang, Z.; Yang, C.; Li, Y. MiR-223/Pknox1 axis protects mice from CVB3-induced viral myocarditis by modulating macrophage polarization. Exp. Cell Res. 2018, 366, 41–48. [Google Scholar] [CrossRef]
- Zhuang, G.; Meng, C.; Guo, X.; Cheruku, P.S.; Shi, L.; Xu, H.; Li, H.; Wang, G.; Evans, A.R.; Safe, S.; et al. A novel regulator of macrophage activation: miR-223 in obesity-associated adipose tissue inflammation. Circulation 2012, 125, 2892–2903. [Google Scholar] [CrossRef]
- He, X.; Dong, Z.; Cao, Y.; Wang, H.; Liu, S.; Liao, L.; Jin, Y.; Yuan, L.; Li, B. MSC-Derived Exosome Promotes M2 Polarization and Enhances Cutaneous Wound Healing. Stem Cells Int. 2019, 2019, 7132708. [Google Scholar] [CrossRef]
- Ma, J.; Chen, L.; Zhu, X.; Li, Q.; Hu, L.; Li, H. Mesenchymal stem cell-derived exosomal miR-21a-5p promotes M2 macrophage polarization and reduces macrophage infiltration to attenuate atherosclerosis. Acta Biochim. Biophys. Sin. 2021, 53, 1227–1236. [Google Scholar] [CrossRef]
- Shen, D.; He, Z. Mesenchymal stem cell-derived exosomes regulate the polarization and inflammatory response of macrophages via miR-21-5p to promote repair after myocardial reperfusion injury. Ann. Transl. Med. 2021, 9, 1323. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Hou, J.; Yang, C.; Wang, H.; Wu, S.; Wu, Y.; Zhao, X.; Lu, C. Extracellular vesicles secreted by hypoxia pre-challenged mesenchymal stem cells promote non-small cell lung cancer cell growth and mobility as well as macrophage M2 polarization via miR-21-5p delivery. J. Exp. Clin. Cancer Res. 2019, 38, 62. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Cai, S.; Wu, M.; Wang, R.; Liu, M.; Cao, G.; Dong, M.; Yiu, K.H. Role of Cardiomyocyte-Derived Exosomal MicroRNA-146a-5p in Macrophage Polarization and Activation. Dis. Markers 2022, 2022, 2948578. [Google Scholar] [CrossRef] [PubMed]
- Altesha, M.A.; Ni, T.; Khan, A.; Liu, K.; Zheng, X. Circular RNA in cardiovascular disease. J. Cell. Physiol. 2019, 234, 5588–5600. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Tu, Z.; Yang, D.; Hu, M.; Zhou, L.; Li, Q.; Yu, B.; Hou, S. Exosomes from hypoxic pre-treated ADSCs attenuate acute ischemic stroke-induced brain injury via delivery of circ-Rps5 and promote M2 microglia/macrophage polarization. Neurosci. Lett. 2022, 769, 136389. [Google Scholar] [CrossRef]
- Devaux, Y.; Zangrando, J.; Schroen, B.; Creemers, E.E.; Pedrazzini, T.; Chang, C.P.; Dorn, G.W., 2nd; Thum, T.; Heymans, S. Long noncoding RNAs in cardiac development and ageing. Nat. Rev. Cardiol. 2015, 12, 415–425. [Google Scholar] [CrossRef]
- Ahmad, I.; Valverde, A.; Naqvi, R.A.; Naqvi, A.R. Long Non-coding RNAs RN7SK and GAS5 Regulate Macrophage Polarization and Innate Immune Responses. Front. Immunol. 2020, 11, 604981. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, W.; Cui, B.; Gao, J.; Liu, Q.; Yao, M.; Ning, H.; Xing, L. Functional delivery of lncRNA TUG1 by endothelial progenitor cells derived extracellular vesicles confers anti-inflammatory macrophage polarization in sepsis via impairing miR-9-5p-targeted SIRT1 inhibition. Cell Death Dis. 2021, 12, 1056. [Google Scholar] [CrossRef]
- Cao, F.; Li, Z.; Ding, W.; Yan, L.; Zhao, Q. Angiotensin II-Treated Cardiac Myocytes Regulate M1 Macrophage Polarization via Transferring Exosomal PVT1. J. Immunol. Res. 2021, 2021, 1994328. [Google Scholar] [CrossRef]
- Hu, D.; Wang, Y.; You, Z.; Lu, Y.; Liang, C. lnc-MRGPRF-6:1 Promotes M1 Polarization of Macrophage and Inflammatory Response through the TLR4-MyD88-MAPK Pathway. Mediat. Inflamm. 2022, 2022, 6979117. [Google Scholar] [CrossRef]
- Liu, J.; Niu, Z.; Zhang, R.; Peng, Z.; Wang, L.; Liu, Z.; Gao, Y.; Pei, H.; Pan, L. MALAT1 shuttled by extracellular vesicles promotes M1 polarization of macrophages to induce acute pancreatitis via miR-181a-5p/HMGB1 axis. J. Cell. Mol. Med. 2021, 25, 9241–9254. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Han, J.; Wu, Y.; Li, S.; Wang, Q.; Lin, W.; Zhu, J. Exosomal MALAT1 derived from oxidized low-density lipoprotein-treated endothelial cells promotes M2 macrophage polarization. Mol. Med. Rep. 2018, 18, 509–515. [Google Scholar] [CrossRef]
- Hansson, G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.J.; Li, Y.S.; Wu, C.C.; Wang, K.C.; Huang, T.C.; Chen, Z.; Chien, S. Extracellular MicroRNA-92a Mediates Endothelial Cell-Macrophage Communication. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 2492–2504. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, W.S.; Xu, L.; Wang, X.; Li, X.L.; Yang, X.C. Endothelium-specific endothelin-1 expression promotes pro-inflammatory macrophage activation by regulating miR-33/NR4A axis. Exp. Cell Res. 2021, 399, 112443. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Wang, X.; Liu, X.; Du, H.; Sun, C.; Shao, X.; Tian, J.; Gu, X.; Wang, H.; Tian, J.; et al. Adipose-Derived Exosomes Exert Proatherogenic Effects by Regulating Macrophage Foam Cell Formation and Polarization. J. Am. Heart Assoc. 2018, 7, e007442. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, Y.; Lin, X.; Zhang, D.; Hu, C.; Liu, J.; Zhu, Y.; Gao, A.; Han, H.; Chai, M.; et al. Perivascular adipose-derived exosomes reduce macrophage foam cell formation through miR-382-5p and the BMP4-PPARγ-ABCA1/ABCG1 pathways. Vasc. Pharmacol. 2022, 143, 106968. [Google Scholar] [CrossRef]
- Takafuji, Y.; Hori, M.; Mizuno, T.; Harada-Shiba, M. Humoral factors secreted from adipose tissue-derived mesenchymal stem cells ameliorate atherosclerosis in Ldlr−/− mice. Cardiovasc. Res. 2019, 115, 1041–1051. [Google Scholar] [CrossRef]
- Li, J.; Xue, H.; Li, T.; Chu, X.; Xin, D.; Xiong, Y.; Qiu, W.; Gao, X.; Qian, M.; Xu, J.; et al. Exosomes derived from mesenchymal stem cells attenuate the progression of atherosclerosis in ApoE−/− mice via miR-let7 mediated infiltration and polarization of M2 macrophage. Biochem. Biophys. Res. Commun. 2019, 510, 565–572. [Google Scholar] [CrossRef]
- Stamatikos, A.; Knight, E.; Vojtech, L.; Bi, L.; Wacker, B.K.; Tang, C.; Dichek, D.A. Exosome-Mediated Transfer of Anti-miR-33a-5p from Transduced Endothelial Cells Enhances Macrophage and Vascular Smooth Muscle Cell Cholesterol Efflux. Hum. Gene Ther. 2020, 31, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Neri, M.; Riezzo, I.; Pascale, N.; Pomara, C.; Turillazzi, E. Ischemia/Reperfusion Injury following Acute Myocardial Infarction: A Critical Issue for Clinicians and Forensic Pathologists. Mediat. Inflamm. 2017, 2017, 7018393. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chen, C.; Spanos, M.; Li, G.; Lu, R.; Bei, Y.; Xiao, J. Exercise training maintains cardiovascular health: Signaling pathways involved and potential therapeutics. Signal Transduct. Target. Ther. 2022, 7, 306. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Meng, Q.; Wei, L.; Liu, J.; Li, M.; Liang, X.; Lin, F.; Zhang, Y.; Li, Y.; Liu, Z.; et al. Myocardial ischemia-reperfusion induced cardiac extracellular vesicles harbour proinflammatory features and aggravate heart injury. J. Extracell. Vesicles 2021, 10, e12072. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xu, Y.; Cao, C.; Wang, B.; Guo, J.; Qin, Z.; Lu, Y.; Zhang, J.; Zhang, L.; Wang, W.; et al. Exosomes as a messager to regulate the crosstalk between macrophages and cardiomyocytes under hypoxia conditions. J. Cell. Mol. Med. 2022, 26, 1486–1500. [Google Scholar] [CrossRef]
- Almeida Paiva, R.; Martins-Marques, T.; Jesus, K.; Ribeiro-Rodrigues, T.; Zuzarte, M.; Silva, A.; Reis, L.; da Silva, M.; Pereira, P.; Vader, P.; et al. Ischaemia alters the effects of cardiomyocyte-derived extracellular vesicles on macrophage activation. J. Cell. Mol. Med. 2019, 23, 1137–1151. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, C.; Liu, L.; A, X.; Chen, B.; Li, Y.; Du, J. Macrophage-Derived mir-155-Containing Exosomes Suppress Fibroblast Proliferation and Promote Fibroblast Inflammation during Cardiac Injury. Mol. Ther. 2017, 25, 192–204. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, R.; Liang, Y.; Fu, X.; Wang, D.; Wang, C. Blockade of lncRNA-ASLNCS5088-enriched exosome generation in M2 macrophages by GW4869 dampens the effect of M2 macrophages on orchestrating fibroblast activation. FASEB J. 2019, 33, 12200–12212. [Google Scholar] [CrossRef]
- Zhao, J.; Li, X.; Hu, J.; Chen, F.; Qiao, S.; Sun, X.; Gao, L.; Xie, J.; Xu, B. Mesenchymal stromal cell-derived exosomes attenuate myocardial ischaemia-reperfusion injury through miR-182-regulated macrophage polarization. Cardiovasc. Res. 2019, 115, 1205–1216. [Google Scholar] [CrossRef]
- Deng, S.; Zhou, X.; Ge, Z.; Song, Y.; Wang, H.; Liu, X.; Zhang, D. Exosomes from adipose-derived mesenchymal stem cells ameliorate cardiac damage after myocardial infarction by activating S1P/SK1/S1PR1 signaling and promoting macrophage M2 polarization. Int. J. Biochem. Cell Biol. 2019, 114, 105564. [Google Scholar] [CrossRef]
- Zhu, F.; Chen, Y.; Li, J.; Yang, Z.; Lin, Y.; Jiang, B.; Shao, L.; Hu, S.; Shen, Z. Human Umbilical Cord Mesenchymal Stem Cell-Derived Exosomes Attenuate Myocardial Infarction Injury via miR-24-3p-Promoted M2 Macrophage Polarization. Adv. Biol. 2022, 6, e2200074. [Google Scholar] [CrossRef] [PubMed]
- Lv, K.; Li, Q.; Zhang, L.; Wang, Y.; Zhong, Z.; Zhao, J.; Lin, X.; Wang, J.; Zhu, K.; Xiao, C.; et al. Incorporation of small extracellular vesicles in sodium alginate hydrogel as a novel therapeutic strategy for myocardial infarction. Theranostics 2019, 9, 7403–7416. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Zhang, F.; Chai, R.; Zhou, W.; Hu, M.; Liu, B.; Chen, X.; Liu, M.; Xu, Q.; Liu, N.; et al. Exosomes derived from pro-inflammatory bone marrow-derived mesenchymal stem cells reduce inflammation and myocardial injury via mediating macrophage polarization. J. Cell. Mol. Med. 2019, 23, 7617–7631. [Google Scholar] [CrossRef] [PubMed]
- Ning, H.; Chen, H.; Deng, J.; Xiao, C.; Xu, M.; Shan, L.; Yang, C.; Zhang, Z. Exosomes secreted by FNDC5-BMMSCs protect myocardial infarction by anti-inflammation and macrophage polarization via NF-κB signaling pathway and Nrf2/HO-1 axis. Stem Cell Res. Ther. 2021, 12, 519. [Google Scholar] [CrossRef]
- Chachques, J.C.; Gardin, C.; Lila, N.; Ferroni, L.; Migonney, V.; Falentin-Daudre, C.; Zanotti, F.; Trentini, M.; Brunello, G.; Rocca, T.; et al. Elastomeric Cardiowrap Scaffolds Functionalized with Mesenchymal Stem Cells-Derived Exosomes Induce a Positive Modulation in the Inflammatory and Wound Healing Response of Mesenchymal Stem Cell and Macrophage. Biomedicines 2021, 9, 824. [Google Scholar] [CrossRef]
- Wang, J.; Lee, C.J.; Deci, M.B.; Jasiewicz, N.; Verma, A.; Canty, J.M.; Nguyen, J. MiR-101a loaded extracellular nanovesicles as bioactive carriers for cardiac repair. Nanomedicine 2020, 27, 102201. [Google Scholar] [CrossRef]
- Mentkowski, K.I.; Mursleen, A.; Snitzer, J.D.; Euscher, L.M.; Lang, J.K. CDC-derived extracellular vesicles reprogram inflammatory macrophages to an arginase 1-dependent proangiogenic phenotype. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H1447–H1460. [Google Scholar] [CrossRef]
- Cambier, L.; de Couto, G.; Ibrahim, A.; Echavez, A.K.; Valle, J.; Liu, W.; Kreke, M.; Smith, R.R.; Marbán, L.; Marbán, E. Y RNA fragment in extracellular vesicles confers cardioprotection via modulation of IL-10 expression and secretion. EMBO Mol. Med. 2017, 9, 337–352. [Google Scholar] [CrossRef]
- Ramanujam, D.; Schon, A.P.; Beck, C.; Vaccarello, P.; Felician, G.; Dueck, A.; Esfandyari, D.; Meister, G.; Meitinger, T.; Schulz, C.; et al. MicroRNA-21-Dependent Macrophage-to-Fibroblast Signaling Determines the Cardiac Response to Pressure Overload. Circulation 2021, 143, 1513–1525. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, W.; Wang, H.; Tang, F.; Zhu, Y.; Zhu, Q.; Ma, R.; Jian, Z.; Xiao, Y. Hypoxia-primed monocytes/macrophages enhance postinfarction myocardial repair. Theranostics 2022, 12, 307–323. [Google Scholar] [CrossRef]
- Sun, X.; Shan, A.; Wei, Z.; Xu, B. Intravenous mesenchymal stem cell-derived exosomes ameliorate myocardial inflammation in the dilated cardiomyopathy. Biochem. Biophys. Res. Commun. 2018, 503, 2611–2618. [Google Scholar] [CrossRef] [PubMed]
- Singla, D.K.; Johnson, T.A.; Tavakoli Dargani, Z. Exosome Treatment Enhances Anti-Inflammatory M2 Macrophages and Reduces Inflammation-Induced Pyroptosis in Doxorubicin-Induced Cardiomyopathy. Cells 2019, 8, 1224. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Qin, L.; Peng, Y.; Bai, W.; Wang, Z. Exosomes Derived From Hypertrophic Cardiomyocytes Induce Inflammation in Macrophages via miR-155 Mediated MAPK Pathway. Front. Immunol. 2020, 11, 606045. [Google Scholar] [CrossRef]
- Lin, Y.N.; Mesquita, T.; Sanchez, L.; Chen, Y.H.; Liu, W.; Li, C.; Rogers, R.; Wang, Y.; Li, X.; Wu, D.; et al. Extracellular vesicles from immortalized cardiosphere-derived cells attenuate arrhythmogenic cardiomyopathy in desmoglein-2 mutant mice. Eur. Heart J. 2021, 42, 3558–3571. [Google Scholar] [CrossRef]
- Xia, W.; Chen, H.; Chen, D.; Ye, Y.; Xie, C.; Hou, M. PD-1 inhibitor inducing exosomal miR-34a-5p expression mediates the cross talk between cardiomyocyte and macrophage in immune checkpoint inhibitor-related cardiac dysfunction. J. Immunother. Cancer 2020, 8, e001293. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Luo, J.; Li, Y.; Jia, L.; Li, Y.; Ye, S.; Liu, L.; Yu, Y.; Lu, Y.; Luan, Y. Macrophage-Derived Exosomes in TLR9−/− Mice Ameliorate Sepsis-Induced Mitochondrial Oxidative Stress and Apoptosis in Cardiomyocytes. Oxid. Med. Cell. Longev. 2022, 2022, 5719974. [Google Scholar] [CrossRef]
- Chen, X.S.; Wang, S.H.; Liu, C.Y.; Gao, Y.L.; Meng, X.L.; Wei, W.; Shou, S.T.; Liu, Y.C.; Chai, Y.F. Losartan attenuates sepsis-induced cardiomyopathy by regulating macrophage polarization via TLR4-mediated NF-kappaB and MAPK signaling. Pharmacol. Res. 2022, 185, 106473. [Google Scholar] [CrossRef]
- Lima Correa, B.; El Harane, N.; Gomez, I.; Rachid Hocine, H.; Vilar, J.; Desgres, M.; Bellamy, V.; Keirththana, K.; Guillas, C.; Perotto, M.; et al. Extracellular vesicles from human cardiovascular progenitors trigger a reparative immune response in infarcted hearts. Cardiovasc. Res. 2021, 117, 292–307. [Google Scholar] [CrossRef]
- de Couto, G.; Gallet, R.; Cambier, L.; Jaghatspanyan, E.; Makkar, N.; Dawkins, J.F.; Berman, B.P.; Marbán, E. Exosomal MicroRNA Transfer Into Macrophages Mediates Cellular Postconditioning. Circulation 2017, 136, 200–214. [Google Scholar] [CrossRef]
- Huang, F.; Na, N.; Ijichi, T.; Wu, X.; Miyamoto, K.; Ciullo, A.; Tran, M.; Li, L.; Ibrahim, A.; Marbán, E.; et al. Exosomally derived Y RNA fragment alleviates hypertrophic cardiomyopathy in transgenic mice. Mol. Ther. Nucleic Acids 2021, 24, 951–960. [Google Scholar] [CrossRef]
- Jia, G.; Hill, M.A.; Sowers, J.R. Diabetic Cardiomyopathy: An Update of Mechanisms Contributing to This Clinical Entity. Circ. Res. 2018, 122, 624–638. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, R.E.; Krum, H. Heart failure in diabetes: Effects of anti-hyperglycaemic drug therapy. Lancet 2015, 385, 2107–2117. [Google Scholar] [CrossRef] [PubMed]
- Mouton, A.J.; Li, X.; Hall, M.E.; Hall, J.E. Obesity, Hypertension, and Cardiac Dysfunction: Novel Roles of Immunometabolism in Macrophage Activation and Inflammation. Circ. Res. 2020, 126, 789–806. [Google Scholar] [CrossRef]
- Elmadbouh, I.; Singla, D.K. BMP-7 Attenuates Inflammation-Induced Pyroptosis and Improves Cardiac Repair in Diabetic Cardiomyopathy. Cells 2021, 10, 2640. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Yan, D.; Yang, L.; Sun, Y.; Zhan, L.; Lu, L.; Jin, Z.; Zhang, C.; Long, P.; Chen, J.; et al. The effect of miR-471-3p on macrophage polarization in the development of diabetic cardiomyopathy. Life Sci. 2021, 268, 118989. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, P.; Kalani, A.; Medina, I.; Familtseva, A.; Tyagi, S.C. Cardiosome mediated regulation of MMP9 in diabetic heart: Role of mir29b and mir455 in exercise. J. Cell. Mol. Med. 2015, 19, 2153–2161. [Google Scholar] [CrossRef]
- Govindappa, P.K.; Patil, M.; Garikipati, V.N.S.; Verma, S.K.; Saheera, S.; Narasimhan, G.; Zhu, W.; Kishore, R.; Zhang, J.; Krishnamurthy, P. Targeting exosome-associated human antigen R attenuates fibrosis and inflammation in diabetic heart. FASEB J. 2020, 34, 2238–2251. [Google Scholar] [CrossRef]
- Li, Q.; Li, Y.; Huang, W.; Wang, X.; Liu, Z.; Chen, J.; Fan, Y.; Peng, T.; Sadayappan, S.; Wang, Y.; et al. Loss of Lipocalin 10 Exacerbates Diabetes-Induced Cardiomyopathy via Disruption of Nr4a1-Mediated Anti-Inflammatory Response in Macrophages. Front. Immunol. 2022, 13, 930397. [Google Scholar] [CrossRef]
- Sagar, S.; Liu, P.P.; Cooper, L.T., Jr. Myocarditis. Lancet 2012, 379, 738–747. [Google Scholar] [CrossRef]
- Westermann, D.; Savvatis, K.; Schultheiss, H.P.; Tschöpe, C. Immunomodulation and matrix metalloproteinases in viral myocarditis. J. Mol. Cell. Cardiol. 2010, 48, 468–473. [Google Scholar] [CrossRef]
- Cheung, C.; Luo, H.; Yanagawa, B.; Leong, H.S.; Samarasekera, D.; Lai, J.C.; Suarez, A.; Zhang, J.; McManus, B.M. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in coxsackievirus-induced myocarditis. Cardiovasc. Pathol. 2006, 15, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Lin, C.; Cui, L.J.; Deng, T.Z.; Li, Q.M.; Chen, F.Y.; Miao, X.P. Mechanism of M2 macrophage-derived extracellular vesicles carrying lncRNA MEG3 in inflammatory responses in ulcerative colitis. Bioengineered 2021, 12, 12722–12739. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.L.; Zhang, S.X.; Zheng, C.F.; Li, Y.F.; Zhang, L.H.; Su, Q.Y.; Hao, Y.F.; Wang, S.; Li, X.W. Long non-coding RNA MEG3 inhibits M2 macrophage polarization by activating TRAF6 via microRNA-223 down-regulation in viral myocarditis. J. Cell. Mol. Med. 2020, 24, 12341–12354. [Google Scholar] [CrossRef]
- Chen, J.; Zheng, J.; Zheng, N.; Zhou, R.; Huang, L.; He, J.; Zhu, W.; Zhang, R. miR-19b-3p/PKNOX1 Regulates Viral Myocarditis by Regulating Macrophage Polarization. Front. Genet. 2022, 13, 902453. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Wang, C.; Zhang, M.; Yang, H.; Lv, K. lncRNA AK085865 Promotes Macrophage M2 Polarization in CVB3-Induced VM by Regulating ILF2-ILF3 Complex-Mediated miRNA-192 Biogenesis. Mol. Ther. Nucleic Acids 2020, 21, 441–451. [Google Scholar] [CrossRef]
- Valaperti, A.; Nishii, M.; Liu, Y.; Naito, K.; Chan, M.; Zhang, L.; Skurk, C.; Schultheiss, H.P.; Wells, G.A.; Eriksson, U.; et al. Innate immune interleukin-1 receptor-associated kinase 4 exacerbates viral myocarditis by reducing CCR5+ CD11b+ monocyte migration and impairing interferon production. Circulation 2013, 128, 1542–1554. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.; Martinez-Pomares, L. Physiological roles of macrophages. Pflug. Arch. 2017, 469, 365–374. [Google Scholar] [CrossRef]
- Pitt, J.M.; André, F.; Amigorena, S.; Soria, J.C.; Eggermont, A.; Kroemer, G.; Zitvogel, L. Dendritic cell-derived exosomes for cancer therapy. J. Clin. Investig. 2016, 126, 1224–1232. [Google Scholar] [CrossRef]
- Wu, R.; Gao, W.; Yao, K.; Ge, J. Roles of Exosomes Derived From Immune Cells in Cardiovascular Diseases. Front. Immunol. 2019, 10, 648. [Google Scholar] [CrossRef]
- Hu, H.; Wu, J.; Cao, C.; Ma, L. Exosomes derived from regulatory T cells ameliorate acute myocardial infarction by promoting macrophage M2 polarization. IUBMB Life 2020, 72, 2409–2419. [Google Scholar] [CrossRef]
- Boilard, E. Extracellular vesicles and their content in bioactive lipid mediators: More than a sack of microRNA. J. Lipid Res. 2018, 59, 2037–2046. [Google Scholar] [CrossRef] [PubMed]
- Eken, C.; Martin, P.J.; Sadallah, S.; Treves, S.; Schaller, M.; Schifferli, J.A. Ectosomes released by polymorphonuclear neutrophils induce a MerTK-dependent anti-inflammatory pathway in macrophages. J. Biol. Chem. 2010, 285, 39914–39921. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, Y.; Wu, W.; Wei, B.; Qin, L. B Cells Increase Myocardial Inflammation by Suppressing M2 Macrophage Polarization in Coxsackie Virus B3-Induced Acute Myocarditis. Inflammation 2019, 42, 953–960. [Google Scholar] [CrossRef]
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Liejendekker, R.; Harding, C.V.; Melief, C.J.; Geuze, H.J. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 1996, 183, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Hood, J.L. The association of exosomes with lymph nodes. Semin. Cell Dev. Biol. 2017, 67, 29–38. [Google Scholar] [CrossRef]
- Lindenbergh, M.F.S.; Stoorvogel, W. Antigen Presentation by Extracellular Vesicles from Professional Antigen-Presenting Cells. Annu. Rev. Immunol. 2018, 36, 435–459. [Google Scholar] [CrossRef] [PubMed]
- Dang, G.; Li, T.; Yang, D.; Yang, G.; Du, X.; Yang, J.; Miao, Y.; Han, L.; Ma, X.; Song, Y.; et al. T lymphocyte-derived extracellular vesicles aggravate abdominal aortic aneurysm by promoting macrophage lipid peroxidation and migration via pyruvate kinase muscle isozyme 2. Redox Biol. 2022, 50, 102257. [Google Scholar] [CrossRef]
- Rolski, F.; Czepiel, M.; Tkacz, K.; Fryt, K.; Siedlar, M.; Kania, G.; Błyszczuk, P. T Lymphocyte-Derived Exosomes Transport MEK1/2 and ERK1/2 and Induce NOX4-Dependent Oxidative Stress in Cardiac Microvascular Endothelial Cells. Oxid. Med. Cell. Longev. 2022, 2022, 2457687. [Google Scholar] [CrossRef]
- Zakharova, L.; Svetlova, M.; Fomina, A.F. T cell exosomes induce cholesterol accumulation in human monocytes via phosphatidylserine receptor. J. Cell. Physiol. 2007, 212, 174–181. [Google Scholar] [CrossRef]
- Hu, H.; Qi, L.; Ren, C.; Yan, S. M2 Macrophage-Derived Exosomes Regulate Myocardial Ischemia-Reperfusion And Pyroptosis Via ROS/NLRP3 Pathway. Heart Surg. Forum 2022, 25, E698–E708. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, S.; Chang, S.; Ren, D.; Shali, S.; Li, C.; Yang, H.; Huang, Z.; Ge, J. M2 macrophage-derived exosomes carry microRNA-148a to alleviate myocardial ischemia/reperfusion injury via inhibiting TXNIP and the TLR4/NF-κB/NLRP3 inflammasome signaling pathway. J. Mol. Cell. Cardiol. 2020, 142, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Long, R.; Gao, L.; Li, Y.; Li, G.; Qin, P.; Wei, Z.; Li, D.; Qian, C.; Li, J.; Yang, G. M2 macrophage-derived exosomes carry miR-1271-5p to alleviate cardiac injury in acute myocardial infarction through down-regulating SOX6. Mol. Immunol. 2021, 136, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Liang, X.; Liu, Y.; Wang, H. Mechanism of miR-378a-3p enriched in M2 macrophage-derived extracellular vesicles in cardiomyocyte pyroptosis after MI. Hypertens. Res. 2022, 45, 650–664. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Liu, Y.; Wang, J.; Zhang, M.; Wang, M. Cardioprotection of M2 macrophages-derived exosomal microRNA-24-3p/Tnfsf10 axis against myocardial injury after sepsis. Mol. Immunol. 2022, 141, 309–317. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Zhao, R.; Qiu, Z.; Shen, C.; Wang, Z.; Liu, W.; Zhang, W.; Ge, J.; Shi, B. CircUbe3a from M2 macrophage-derived small extracellular vesicles mediates myocardial fibrosis after acute myocardial infarction. Theranostics 2021, 11, 6315–6333. [Google Scholar] [CrossRef]
- Salybekov, A.A.; Salybekova, A.; Sheng, Y.; Shinozaki, Y.; Yokoyama, K.; Kobayashi, S.; Asahara, T. Extracellular Vesicles Derived From Regeneration Associated Cells Preserve Heart Function After Ischemia-Induced Injury. Front. Cardiovasc. Med. 2021, 8, 754254. [Google Scholar] [CrossRef]
- Bouchareychas, L.; Duong, P.; Covarrubias, S.; Alsop, E.; Phu, T.A.; Chung, A.; Gomes, M.; Wong, D.; Meechoovet, B.; Capili, A.; et al. Macrophage Exosomes Resolve Atherosclerosis by Regulating Hematopoiesis and Inflammation via MicroRNA Cargo. Cell Rep. 2020, 32, 107881. [Google Scholar] [CrossRef]
- Cheng, X.; Zhou, H.; Zhou, Y.; Song, C. M2 Macrophage-Derived Exosomes Inhibit Apoptosis of HUVEC Cell through Regulating miR-221-3p Expression. Biomed. Res. Int. 2022, 2022, 1609244. [Google Scholar] [CrossRef]
- Liang, W.; Chen, J.; Zheng, H.; Lin, A.; Li, J.; Wu, W.; Jie, Q. MiR-199a-5p-containing macrophage-derived extracellular vesicles inhibit SMARCA4 and alleviate atherosclerosis by reducing endothelial cell pyroptosis. Cell Biol. Toxicol. 2022. [Google Scholar] [CrossRef]
- Liu, P.; Wang, S.; Wang, G.; Zhao, M.; Du, F.; Li, K.; Wang, L.; Wu, H.; Chen, J.; Yang, Y.; et al. Macrophage-derived exosomal miR-4532 promotes endothelial cells injury by targeting SP1 and NF-κB P65 signalling activation. J. Cell. Mol. Med. 2022, 26, 5165–5180. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Z.; Wang, X.; Zheng, J.; Peng, L.; Zhou, Y.; Song, Y.; Lu, Z. Extracellular-vesicle containing miRNA-503-5p released by macrophages contributes to atherosclerosis. Aging 2021, 13, 12239–12257. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Chen, S.; Yao, D.; Yan, H. OxLDL-stimulated macrophage exosomes promote proatherogenic vascular smooth muscle cell viability and invasion via delivering miR-186-5p then inactivating SHIP2 mediated PI3K/AKT/mTOR pathway. Mol. Immunol. 2022, 146, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, W.L.; Gu, J.J.; Sun, Y.Q.; Cui, H.Z.; Bu, J.Q.; Chen, Z.Y. Exosome-mediated miR-106a-3p derived from ox-LDL exposed macrophages accelerated cell proliferation and repressed cell apoptosis of human vascular smooth muscle cells. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 7039–7050. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Dong, Y.; Wang, H. microRNA-19b-3p-containing extracellular vesicles derived from macrophages promote the development of atherosclerosis by targeting JAZF1. J. Cell. Mol. Med. 2022, 26, 48–59. [Google Scholar] [CrossRef]
- Li, K.; Cui, M.; Zhang, K.; Wang, G.; Zhai, S. M1 macrophages-derived extracellular vesicles elevate microRNA-185-3p to aggravate the development of atherosclerosis in ApoE−/− mice by inhibiting small mothers against decapentaplegic 7. Int. Immunopharmacol. 2021, 90, 107138. [Google Scholar] [CrossRef]
- Chen, F.; Li, J.; She, J.; Chen, T.; Yuan, Z. Exosomal microRNA-16-5p from macrophage exacerbates atherosclerosis via modulating mothers against decapentaplegic homolog 7. Microvasc. Res. 2022, 142, 104368. [Google Scholar] [CrossRef]
- Liu, S.; Chen, J.; Shi, J.; Zhou, W.; Wang, L.; Fang, W.; Zhong, Y.; Chen, X.; Chen, Y.; Sabri, A.; et al. M1-like macrophage-derived exosomes suppress angiogenesis and exacerbate cardiac dysfunction in a myocardial infarction microenvironment. Basic Res. Cardiol. 2020, 115, 22. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Z.M.; Ji, J.L.; Gan, W.; Zhang, A.; Shi, H.J.; Wang, H.; Lv, L.; Li, Z.; Tang, T.; et al. Macrophage-Derived Exosomal Mir-155 Regulating Cardiomyocyte Pyroptosis and Hypertrophy in Uremic Cardiomyopathy. JACC Basic Transl. Sci. 2020, 5, 148–166. [Google Scholar] [CrossRef]
- Dong, J.; Zhu, W.; Wan, D. Downregulation of microRNA-21-5p from macrophages-derived exosomes represses ventricular remodeling after myocardial infarction via inhibiting tissue inhibitors of metalloproteinase 3. Int. Immunopharmacol. 2021, 96, 107611. [Google Scholar] [CrossRef]
- Chen, B.; Luo, L.; Wei, X.; Gong, D.; Li, Z.; Li, S.; Tang, W.; Jin, L. M1 Bone Marrow-Derived Macrophage-Derived Extracellular Vesicles Inhibit Angiogenesis and Myocardial Regeneration Following Myocardial Infarction via the MALAT1/MicroRNA-25-3p/CDC42 Axis. Oxid. Med. Cell. Longev. 2021, 2021, 9959746. [Google Scholar] [CrossRef]
- Arandjelovic, S.; Ravichandran, K.S. Phagocytosis of apoptotic cells in homeostasis. Nat. Immunol. 2015, 16, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Szondy, Z.; Garabuczi, E.; Joós, G.; Tsay, G.J.; Sarang, Z. Impaired clearance of apoptotic cells in chronic inflammatory diseases: Therapeutic implications. Front. Immunol. 2014, 5, 354. [Google Scholar] [CrossRef] [PubMed]
- Glinton, K.E.; Ma, W.; Lantz, C.; Grigoryeva, L.S.; DeBerge, M.; Liu, X.; Febbraio, M.; Kahn, M.; Oliver, G.; Thorp, E.B. Macrophage-produced VEGFC is induced by efferocytosis to ameliorate cardiac injury and inflammation. J. Clin. Investig. 2022, 132, e140685. [Google Scholar] [CrossRef]
- Yurdagul, A., Jr.; Doran, A.C.; Cai, B.; Fredman, G.; Tabas, I.A. Mechanisms and Consequences of Defective Efferocytosis in Atherosclerosis. Front. Cardiovasc. Med. 2017, 4, 86. [Google Scholar] [CrossRef]
- Linton, M.F.; Babaev, V.R.; Huang, J.; Linton, E.F.; Tao, H.; Yancey, P.G. Macrophage Apoptosis and Efferocytosis in the Pathogenesis of Atherosclerosis. Circ. J. 2016, 80, 2259–2268. [Google Scholar] [CrossRef] [PubMed]
- Kasikara, C.; Schilperoort, M.; Gerlach, B.; Xue, C.; Wang, X.; Zheng, Z.; Kuriakose, G.; Dorweiler, B.; Zhang, H.; Fredman, G.; et al. Deficiency of macrophage PHACTR1 impairs efferocytosis and promotes atherosclerotic plaque necrosis. J. Clin. Investig. 2021, 131, e145275. [Google Scholar] [CrossRef]
- Bartoli-Leonard, F.; Saddic, L.; Aikawa, E. Double-edged sword of ALDH2 mutations: One polymorphism can both benefit and harm the cardiovascular system. Eur. Heart J. 2020, 41, 2453–2455. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, X.; Guo, Y.; Liu, Z.; Wei, S.; Yuan, Q.; Shang, H.; Sang, W.; Cui, S.; Xu, T.; et al. Macrophage ALDH2 (Aldehyde Dehydrogenase 2) Stabilizing Rac2 Is Required for Efferocytosis Internalization and Reduction of Atherosclerosis Development. Arterioscler. Thromb. Vasc. Biol. 2022, 42, 700–716. [Google Scholar] [CrossRef]
- Monguió-Tortajada, M.; Prat-Vidal, C.; Martínez-Falguera, D.; Teis, A.; Soler-Botija, C.; Courageux, Y.; Munizaga-Larroudé, M.; Moron-Font, M.; Bayes-Genis, A.; Borràs, F.E.; et al. Acellular cardiac scaffolds enriched with MSC-derived extracellular vesicles limit ventricular remodelling and exert local and systemic immunomodulation in a myocardial infarction porcine model. Theranostics 2022, 12, 4656–4670. [Google Scholar] [CrossRef]
- de Almeida Oliveira, N.C.; Neri, E.A.; Silva, C.M.; Valadão, I.C.; Fonseca-Alaniz, M.H.; Zogbi, C.; Levy, D.; Bydlowski, S.P.; Krieger, J.E. Multicellular regulation of miR-196a-5p and miR-425-5 from adipose stem cell-derived exosomes and cardiac repair. Clin. Sci. 2022, 136, 1281–1301. [Google Scholar] [CrossRef]
- Comariţa, I.K.; Vîlcu, A.; Constantin, A.; Procopciuc, A.; Safciuc, F.; Alexandru, N.; Dragan, E.; Nemecz, M.; Filippi, A.; Chiţoiu, L.; et al. Therapeutic Potential of Stem Cell-Derived Extracellular Vesicles on Atherosclerosis-Induced Vascular Dysfunction and Its Key Molecular Players. Front. Cell Dev. Biol. 2022, 10, 817180. [Google Scholar] [CrossRef] [PubMed]
- Bu, T.; Li, Z.; Hou, Y.; Sun, W.; Zhang, R.; Zhao, L.; Wei, M.; Yang, G.; Yuan, L. Exosome-mediated delivery of inflammation-responsive Il-10 mRNA for controlled atherosclerosis treatment. Theranostics 2021, 11, 9988–10000. [Google Scholar] [CrossRef]
- Wu, G.; Zhang, J.; Zhao, Q.; Zhuang, W.; Ding, J.; Zhang, C.; Gao, H.; Pang, D.W.; Pu, K.; Xie, H.Y. Molecularly Engineered Macrophage-Derived Exosomes with Inflammation Tropism and Intrinsic Heme Biosynthesis for Atherosclerosis Treatment. Angew. Chem. Int. Ed. Engl. 2020, 59, 4068–4074. [Google Scholar] [CrossRef]
- Wei, Z.; Chen, Z.; Zhao, Y.; Fan, F.; Xiong, W.; Song, S.; Yin, Y.; Hu, J.; Yang, K.; Yang, L.; et al. Mononuclear phagocyte system blockade using extracellular vesicles modified with CD47 on membrane surface for myocardial infarction reperfusion injury treatment. Biomaterials 2021, 275, 121000. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, S.; Giavaresi, G.; Lorico, A.; Alessandro, R. Extracellular Vesicles as Biological Shuttles for Targeted Therapies. Int. J. Mol. Sci. 2019, 20, 1848. [Google Scholar] [CrossRef]
- Raimondo, S.; Urzì, O.; Meraviglia, S.; Di Simone, M.; Corsale, A.M.; Rabienezhad Ganji, N.; Palumbo Piccionello, A.; Polito, G.; Lo Presti, E.; Dieli, F.; et al. Anti-inflammatory properties of lemon-derived extracellular vesicles are achieved through the inhibition of ERK/NF-κB signalling pathways. J. Cell. Mol. Med. 2022, 26, 4195–4209. [Google Scholar] [CrossRef]
- Kim, M.K.; Choi, Y.C.; Cho, S.H.; Choi, J.S.; Cho, Y.W. The Antioxidant Effect of Small Extracellular Vesicles Derived from Aloe vera Peels for Wound Healing. Tissue Eng. Regen. Med. 2021, 18, 561–571. [Google Scholar] [CrossRef]
- Kim, M.; Park, J.H. Isolation of Aloe saponaria-Derived Extracellular Vesicles and Investigation of Their Potential for Chronic Wound Healing. Pharmaceutics 2022, 14, 1905. [Google Scholar] [CrossRef]
- Savcı, Y.; Kırbaş, O.K.; Bozkurt, B.T.; Abdik, E.A.; Taşlı, P.N.; Şahin, F.; Abdik, H. Grapefruit-derived extracellular vesicles as a promising cell-free therapeutic tool for wound healing. Food Funct. 2021, 12, 5144–5156. [Google Scholar] [CrossRef]
- Mu, J.; Zhuang, X.; Wang, Q.; Jiang, H.; Deng, Z.B.; Wang, B.; Zhang, L.; Kakar, S.; Jun, Y.; Miller, D.; et al. Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol. Nutr. Food Res. 2014, 58, 1561–1573. [Google Scholar] [CrossRef] [PubMed]
- Puddu, P.; Puddu, G.M.; Cravero, E.; Muscari, S.; Muscari, A. The involvement of circulating microparticles in inflammation, coagulation and cardiovascular diseases. Can. J. Cardiol. 2010, 26, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, Y.; Zhu, Q.; Zhao, J.; Wang, Y.; Shang, M.; Liu, M.; Wu, Y.; Song, J.; Liu, Y. Protective effects of circulating microvesicles derived from ischemic preconditioning on myocardial ischemia/reperfusion injury in rats by inhibiting endoplasmic reticulum stress. Apoptosis 2018, 23, 436–448. [Google Scholar] [CrossRef] [PubMed]
- Giannopoulos, G.; Oudatzis, G.; Paterakis, G.; Synetos, A.; Tampaki, E.; Bouras, G.; Hahalis, G.; Alexopoulos, D.; Tousoulis, D.; Cleman, M.W.; et al. Red blood cell and platelet microparticles in myocardial infarction patients treated with primary angioplasty. Int. J. Cardiol. 2014, 176, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.T.; Wang, B.; Li, Z.L.; Wen, Y.; Feng, S.T.; Wu, M.; Liu, D.; Cao, J.Y.; Yin, Q.; Yin, D.; et al. Kim-1 Targeted Extracellular Vesicles: A New Therapeutic Platform for RNAi to Treat AKI. J. Am. Soc. Nephrol. 2021, 32, 2467–2483. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Jin, H.; Tan, H.; Cai, X.; Sun, Y. Erythrocyte-derived extracellular vesicles aggravate inflammation by promoting the proinflammatory macrophage phenotype through TLR4-MyD88-NF-κB-MAPK pathway. J. Leukoc. Biol. 2022, 112, 693–706. [Google Scholar] [CrossRef]
- Wang, W.Y.; Zheng, Y.S.; Li, Z.G.; Cui, Y.M.; Jiang, J.C. MiR-92a contributes to the cardiovascular disease development in diabetes mellitus through NF-kappaB and downstream inflammatory pathways. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 3070–3079. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, J.; Chen, F.; Wu, C.; Zhang, J.; Ren, X.; Pan, Y.; Nie, B.; Li, Q.; Li, Y. Circulating endothelial microparticles and miR-92a in acute myocardial infarction. Biosci. Rep. 2017, 37, BSR20170047. [Google Scholar] [CrossRef]
- Niculescu, L.S.; Simionescu, N.; Sanda, G.M.; Carnuta, M.G.; Stancu, C.S.; Popescu, A.C.; Popescu, M.R.; Vlad, A.; Dimulescu, D.R.; Simionescu, M.; et al. MiR-486 and miR-92a Identified in Circulating HDL Discriminate between Stable and Vulnerable Coronary Artery Disease Patients. PLoS ONE 2015, 10, e0140958. [Google Scholar] [CrossRef]
- Huang, Y.; Tang, S.; Ji-Yan, C.; Huang, C.; Li, J.; Cai, A.P.; Feng, Y.Q. Circulating miR-92a expression level in patients with essential hypertension: A potential marker of atherosclerosis. J. Hum. Hypertens. 2017, 31, 200–205. [Google Scholar] [CrossRef]
- Xu, L.; Tian, L.; Yan, Z.; Wang, J.; Xue, T.; Sun, Q. Diagnostic and prognostic value of miR-486-5p, miR-451a, miR-21-5p and monocyte to high-density lipoprotein cholesterol ratio in patients with acute myocardial infarction. Heart Vessels 2023, 38, 318–331. [Google Scholar] [CrossRef]
- Marketou, M.; Kontaraki, J.; Patrianakos, A.; Kochiadakis, G.; Anastasiou, I.; Fragkiadakis, K.; Plevritaki, A.; Papadaki, S.T.; Chlouverakis, G.; Parthenakis, F. Peripheral Blood MicroRNAs as Potential Biomarkers of Myocardial Damage in Acute Viral Myocarditis. Genes 2021, 12, 420. [Google Scholar] [CrossRef]
- Yang, H.; Shan, L.; Gao, Y.; Li, L.; Xu, G.; Wang, B.; Yin, X.; Gao, C.; Liu, J.; Yang, W. MicroRNA-181b Serves as a Circulating Biomarker and Regulates Inflammation in Heart Failure. Dis. Markers 2021, 2021, 4572282. [Google Scholar] [CrossRef]
- Copier, C.U.; Leon, L.; Fernandez, M.; Contador, D.; Calligaris, S.D. Circulating miR-19b and miR-181b are potential biomarkers for diabetic cardiomyopathy. Sci. Rep. 2017, 7, 13514. [Google Scholar] [CrossRef] [PubMed]
- Nahrendorf, M.; Swirski, F.K. Abandoning M1/M2 for a Network Model of Macrophage Function. Circ. Res. 2016, 119, 414–417. [Google Scholar] [CrossRef]
- Yang, K.; Xiao, Q.; Niu, M.; Pan, X.; Zhu, X. Exosomes in atherosclerosis: Convergence on macrophages. Int. J. Biol. Sci. 2022, 18, 3266–3281. [Google Scholar] [CrossRef] [PubMed]
- Holthaus, M.; Santhakumar, N.; Wahlers, T.; Paunel-Gorgulu, A. The Secretome of Preconditioned Mesenchymal Stem Cells Drives Polarization and Reprogramming of M2a Macrophages toward an IL-10-Producing Phenotype. Int. J. Mol. Sci. 2022, 23, 4104. [Google Scholar] [CrossRef]
- Kudlik, G.; Hegyi, B.; Czibula, A.; Monostori, E.; Buday, L.; Uher, F. Mesenchymal stem cells promote macrophage polarization toward M2b-like cells. Exp. Cell Res. 2016, 348, 36–45. [Google Scholar] [CrossRef]
- Wang, L.X.; Zhang, S.X.; Wu, H.J.; Rong, X.L.; Guo, J. M2b macrophage polarization and its roles in diseases. J. Leukoc. Biol. 2019, 106, 345–358. [Google Scholar] [CrossRef] [PubMed]



| Model | Cell of Origin | Contents | Effects | Target Gene/Single Pathway | Reference |
|---|---|---|---|---|---|
| AS | endothelial cells | miR-92a | promote M1 macrophage polarization | suppressed the expression of the target gene KLF4 | [45] |
| AS | endothelial cells | miR-33 | promote pro-inflammatory macrophage activation | targeting to NR4A | [46] |
| AS | perivascular adipose tissue | miR-382-5p | reduce macrophage foam cell formation | BMP4-PPARγ-ABCA1/ABCG1 pathways | [48] |
| AS | adipose tissue | / | induce M1 phenotype and proinflammatory cytokine release | increased phosphorylation of NF-κB-p65 | [47] |
| AS | adipose tissue-derived MSCs | / | increase the expression of M2 markers | inhibiting MAPK, NF-κB pathways and activating the STAT3 pathway | [49] |
| AS | mesenchymal stem cells | miR-let7 | promote M2 macrophage polarization | HMGA2/NF-κB and IGF2BP1/PTEN pathway | [50] |
| AS | mesenchymal stem cells | miR-21a-5p | promote M2 macrophage polarization | Inhibit KLF6 and ERK1/2 single pathway | [31] |
| MI/MIR | cardiomyocyte | miR-155-5p | facilitate M1 polarization with increased expression of inflammatory cytokines | activating JAK2/STAT1 pathway | [54] |
| MI/MIR | mesenchymal stem cells | miR-182 | modify the polarization of M1 macrophages to M2 macrophages | TLR4 as a downstream target | [59] |
| MI/MIR | mesenchymal stem cells | miR-21-5p | promote M2 macrophage polarization | / | [32] |
| MI/MIR | adipose tissue-derived MSCs | / | promote M2 macrophage polarization | S1P/SK1/S1PR1 pathway | [60] |
| MI/MIR | cardiac progenitor cell | / | reduce M1 macrophages and increase M2 macrophages | / | [78] |
| MI/MIR | Cardio-sphere derived cell | / | increase M1 macrophage polarization | arginase 1 upregulation | [67] |
| MI/MIR | Cardio-sphere derived cell | miR-181b | reduce CD68+ macrophage and modify the polarization state of macrophage | targeting to PKCδ | [79] |
| MI/MIR | Cardio-sphere derived cell | Y RNA | Induce the secretion of IL-10 in macrophages | / | [68] |
| DCM | mesenchymal stem cells | / | reduce M1 macrophages and increase M2 macrophages | JAK2-STAT6 pathway | [71] |
| HCM | Cardio-sphere derived cell | YF1 | decrease proinflammatory monocytes | reverse hypertrophic and fibrotic signaling pathways | [80] |
| HCM | hypertrophic cardiomyocytes | miR-155 | increased expression of proinflammatory cytokines | increased phosphorylation of ERK, JNK and p38 | [73] |
| DIC | embryonic stem cell | / | increase M2 macrophages and IL-10 | / | [72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Ye, X.; Spanos, M.; Wang, H.; Yang, Z.; Li, G.; Xiao, J.; Zhou, L. Exosomal Non-Coding RNA Mediates Macrophage Polarization: Roles in Cardiovascular Diseases. Biology 2023, 12, 745. https://doi.org/10.3390/biology12050745
Wang H, Ye X, Spanos M, Wang H, Yang Z, Li G, Xiao J, Zhou L. Exosomal Non-Coding RNA Mediates Macrophage Polarization: Roles in Cardiovascular Diseases. Biology. 2023; 12(5):745. https://doi.org/10.3390/biology12050745
Chicago/Turabian StyleWang, Hongyun, Xuan Ye, Michail Spanos, Huanxin Wang, Zijiang Yang, Guoping Li, Junjie Xiao, and Lei Zhou. 2023. "Exosomal Non-Coding RNA Mediates Macrophage Polarization: Roles in Cardiovascular Diseases" Biology 12, no. 5: 745. https://doi.org/10.3390/biology12050745
APA StyleWang, H., Ye, X., Spanos, M., Wang, H., Yang, Z., Li, G., Xiao, J., & Zhou, L. (2023). Exosomal Non-Coding RNA Mediates Macrophage Polarization: Roles in Cardiovascular Diseases. Biology, 12(5), 745. https://doi.org/10.3390/biology12050745