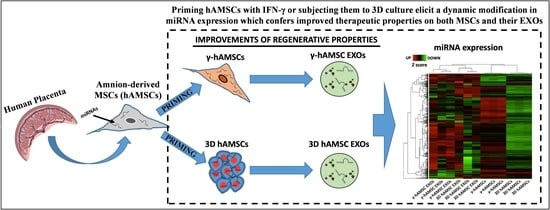
3D Culture and Interferon-γ Priming Modulates Characteristics of Mesenchymal Stromal/Stem Cells by Modifying the Expression of Both Intracellular and Exosomal microRNAs
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Mesenchymal Stromal/Stem Cells from Human Amniotic Membrane
2.2. Priming of hAMSCs with IFN-γ Treatment or by Growing as Spheroids and Conditioned Medium Preparation
2.3. Isolation and Characterization of Exosomes (EXOs)
2.4. Real-Time PCR Analysis of miRNAs with TaqMan Low Density Arrays
2.5. Target Gene Prediction and Gene Ontology (GO) Analysis
2.6. Statistical Analysis
3. Results
3.1. Culture, Characterization, Spheroid Formation of hAMSCs, and EXO Isolation
3.2. Differentially Expressed miRNAs (DEMs) in Primed hAMSCs
3.3. Pathway Analysis of miRNA Targets (GO Biological Process)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal Stem Cells for Regenerative Medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef] [Green Version]
- Margiana, R.; Markov, A.; Zekiy, A.O.; Hamza, M.U.; Al-Dabbagh, K.A.; Al-Zubaidi, S.H.; Hameed, N.M.; Ahmad, I.; Sivaraman, R.; Kzar, H.H.; et al. Clinical application of mesenchymal stem cell in regenerative medicine: A narrative review. Stem Cell Res. Ther. 2022, 13, 366. [Google Scholar] [CrossRef]
- Miceli, V.; Bulati, M.; Gallo, A.; Iannolo, G.; Busa, R.; Conaldi, P.G.; Zito, G. Role of Mesenchymal Stem/Stromal Cells in Modulating Ischemia/Reperfusion Injury: Current State of the Art and Future Perspectives. Biomedicines 2023, 11, 689. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Yan, J.; Yao, Z.; Zhang, C.; Li, X.; Mao, H.Q. Effects of Mesenchymal Stem Cell-Derived Paracrine Signals and Their Delivery Strategies. Adv. Healthc. Mater. 2021, 10, e2001689. [Google Scholar] [CrossRef] [PubMed]
- Miceli, V.; Zito, G.; Bulati, M.; Gallo, A.; Busa, R.; Iannolo, G.; Conaldi, P.G. Different priming strategies improve distinct therapeutic capabilities of mesenchymal stromal/stem cells: Potential implications for their clinical use. World J. Stem Cells 2023, 15, 400–420. [Google Scholar] [CrossRef] [PubMed]
- Nikfarjam, S.; Rezaie, J.; Zolbanin, N.M.; Jafari, R. Mesenchymal stem cell derived-exosomes: A modern approach in translational medicine. J. Transl. Med. 2020, 18, 449. [Google Scholar] [CrossRef]
- Sohrabi, B.; Dayeri, B.; Zahedi, E.; Khoshbakht, S.; Nezamabadi Pour, N.; Ranjbar, H.; Davari Nejad, A.; Noureddini, M.; Alani, B. Mesenchymal stem cell (MSC)-derived exosomes as novel vehicles for delivery of miRNAs in cancer therapy. Cancer Gene Ther. 2022, 29, 1105–1116. [Google Scholar] [CrossRef]
- Yao, Q.; Chen, Y.; Zhou, X. The roles of microRNAs in epigenetic regulation. Curr. Opin. Chem. Biol. 2019, 51, 11–17. [Google Scholar] [CrossRef]
- Gupta, S.K.; Bang, C.; Thum, T. Circulating microRNAs as biomarkers and potential paracrine mediators of cardiovascular disease. Circ. Cardiovasc. Genet. 2010, 3, 484–488. [Google Scholar] [CrossRef] [Green Version]
- Martucci, G.; Arcadipane, A.; Tuzzolino, F.; Occhipinti, G.; Panarello, G.; Carcione, C.; Bonicolini, E.; Vitiello, C.; Lorusso, R.; Conaldi, P.G.; et al. Identification of a Circulating miRNA Signature to Stratify Acute Respiratory Distress Syndrome Patients. J. Pers. Med. 2020, 11, 15. [Google Scholar] [CrossRef]
- Sugimachi, K.; Matsumura, T.; Hirata, H.; Uchi, R.; Ueda, M.; Ueo, H.; Shinden, Y.; Iguchi, T.; Eguchi, H.; Shirabe, K.; et al. Identification of a bona fide microRNA biomarker in serum exosomes that predicts hepatocellular carcinoma recurrence after liver transplantation. Br. J. Cancer 2015, 112, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Bilinska, A.; Pszczola, M.; Stachowiak, M.; Stachecka, J.; Garbacz, F.; Aksoy, M.O.; Szczerbal, I. Droplet Digital PCR Quantification of Selected Intracellular and Extracellular microRNAs Reveals Changes in Their Expression Pattern during Porcine In Vitro Adipogenesis. Genes 2023, 14, 683. [Google Scholar] [CrossRef]
- Zou, X.Y.; Yu, Y.; Lin, S.; Zhong, L.; Sun, J.; Zhang, G.; Zhu, Y. Comprehensive miRNA Analysis of Human Umbilical Cord-Derived Mesenchymal Stromal Cells and Extracellular Vesicles. Kidney Blood Press. Res. 2018, 43, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Tavasolian, F.; Hosseini, A.Z.; Soudi, S.; Naderi, M. miRNA-146a Improves Immunomodulatory Effects of MSC-derived Exosomes in Rheumatoid Arthritis. Curr. Gene Ther. 2020, 20, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.K.; Chen, L.J.; Zhou, S.N.; Li, Y.F.; Xiang, C. Multifunctional role of microRNAs in mesenchymal stem cell-derived exosomes in treatment of diseases. World J. Stem Cells 2020, 12, 1276–1294. [Google Scholar] [CrossRef]
- Xue, C.; Li, X.; Ba, L.; Zhang, M.; Yang, Y.; Gao, Y.; Sun, Z.; Han, Q.; Zhao, R.C. MSC-Derived Exosomes can Enhance the Angiogenesis of Human Brain MECs and Show Therapeutic Potential in a Mouse Model of Parkinson’s Disease. Aging Dis. 2021, 12, 1211–1222. [Google Scholar] [CrossRef]
- Gallo, A.; Cuscino, N.; Contino, F.; Bulati, M.; Pampalone, M.; Amico, G.; Zito, G.; Carcione, C.; Centi, C.; Bertani, A.; et al. Changes in the Transcriptome Profiles of Human Amnion-Derived Mesenchymal Stromal/Stem Cells Induced by Three-Dimensional Culture: A Potential Priming Strategy to Improve Their Properties. Int. J. Mol. Sci. 2022, 23, 863. [Google Scholar] [CrossRef]
- Zito, G.; Miceli, V.; Carcione, C.; Busa, R.; Bulati, M.; Gallo, A.; Iannolo, G.; Pagano, D.; Conaldi, P.G. Human Amnion-Derived Mesenchymal Stromal/Stem Cells Pre-Conditioning Inhibits Inflammation and Apoptosis of Immune and Parenchymal Cells in an In Vitro Model of Liver Ischemia/Reperfusion. Cells 2022, 11, 709. [Google Scholar] [CrossRef]
- Lo Nigro, A.; Gallo, A.; Bulati, M.; Vitale, G.; Paini, D.S.; Pampalone, M.; Galvagno, D.; Conaldi, P.G.; Miceli, V. Amnion-Derived Mesenchymal Stromal/Stem Cell Paracrine Signals Potentiate Human Liver Organoid Differentiation: Translational Implications for Liver Regeneration. Front. Med. 2021, 8, 746298. [Google Scholar] [CrossRef]
- Forsberg, M.H.; Kink, J.A.; Thickens, A.S.; Lewis, B.M.; Childs, C.J.; Hematti, P.; Capitini, C.M. Exosomes from primed MSCs can educate monocytes as a cellular therapy for hematopoietic acute radiation syndrome. Stem Cell Res. Ther. 2021, 12, 459. [Google Scholar] [CrossRef]
- Heo, J.S.; Kim, S. Human adipose mesenchymal stem cells modulate inflammation and angiogenesis through exosomes. Sci. Rep. 2022, 12, 2776. [Google Scholar] [CrossRef] [PubMed]
- Kern, F.; Fehlmann, T.; Solomon, J.; Schwed, L.; Grammes, N.; Backes, C.; Van Keuren-Jensen, K.; Craig, D.W.; Meese, E.; Keller, A. miEAA 2.0: Integrating multi-species microRNA enrichment analysis and workflow management systems. Nucleic Acids Res. 2020, 48, W521–W528. [Google Scholar] [CrossRef] [PubMed]
- Backes, C.; Keller, A.; Kuentzer, J.; Kneissl, B.; Comtesse, N.; Elnakady, Y.A.; Muller, R.; Meese, E.; Lenhof, H.P. GeneTrail—Advanced gene set enrichment analysis. Nucleic Acids Res. 2007, 35, W186–W192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benjamini, Y.; Drai, D.; Elmer, G.; Kafkafi, N.; Golani, I. Controlling the false discovery rate in behavior genetics research. Behav. Brain Res. 2001, 125, 279–284. [Google Scholar] [CrossRef] [Green Version]
- Song, N.; Scholtemeijer, M.; Shah, K. Mesenchymal Stem Cell Immunomodulation: Mechanisms and Therapeutic Potential. Trends Pharmacol. Sci. 2020, 41, 653–664. [Google Scholar] [CrossRef]
- Tao, H.; Han, Z.; Han, Z.C.; Li, Z. Proangiogenic Features of Mesenchymal Stem Cells and Their Therapeutic Applications. Stem Cells Int. 2016, 2016, 1314709. [Google Scholar] [CrossRef] [Green Version]
- Merimi, M.; El-Majzoub, R.; Lagneaux, L.; Moussa Agha, D.; Bouhtit, F.; Meuleman, N.; Fahmi, H.; Lewalle, P.; Fayyad-Kazan, M.; Najar, M. The Therapeutic Potential of Mesenchymal Stromal Cells for Regenerative Medicine: Current Knowledge and Future Understandings. Front. Cell Dev. Biol. 2021, 9, 661532. [Google Scholar] [CrossRef]
- Miceli, V.; Bertani, A. Mesenchymal Stromal/Stem Cells and Their Products as a Therapeutic Tool to Advance Lung Transplantation. Cells 2022, 11, 826. [Google Scholar] [CrossRef]
- Schmelzer, E.; Miceli, V.; Chinnici, C.M.; Bertani, A.; Gerlach, J.C. Effects of Mesenchymal Stem Cell Coculture on Human Lung Small Airway Epithelial Cells. BioMed. Res. Int. 2020, 2020, 9847579. [Google Scholar] [CrossRef]
- Fuloria, S.; Subramaniyan, V.; Dahiya, R.; Dahiya, S.; Sudhakar, K.; Kumari, U.; Sathasivam, K.; Meenakshi, D.U.; Wu, Y.S.; Sekar, M.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Regenerative Potential and Challenges. Biology 2021, 10, 172. [Google Scholar] [CrossRef]
- Kim, G.B.; Shon, O.J.; Seo, M.S.; Choi, Y.; Park, W.T.; Lee, G.W. Mesenchymal Stem Cell-Derived Exosomes and Their Therapeutic Potential for Osteoarthritis. Biology 2021, 10, 285. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.C.; Kang, I.; Yu, K.R. Therapeutic Features and Updated Clinical Trials of Mesenchymal Stem Cell (MSC)-Derived Exosomes. J. Clin. Med. 2021, 10, 711. [Google Scholar] [CrossRef] [PubMed]
- Re, F.; Gabusi, E.; Manferdini, C.; Russo, D.; Lisignoli, G. Bone Regeneration Improves with Mesenchymal Stem Cell Derived Extracellular Vesicles (EVs) Combined with Scaffolds: A Systematic Review. Biology 2021, 10, 579. [Google Scholar] [CrossRef] [PubMed]
- Asgarpour, K.; Shojaei, Z.; Amiri, F.; Ai, J.; Mahjoubin-Tehran, M.; Ghasemi, F.; ArefNezhad, R.; Hamblin, M.R.; Mirzaei, H. Exosomal microRNAs derived from mesenchymal stem cells: Cell-to-cell messages. Cell Commun. Signal. CCS 2020, 18, 149. [Google Scholar] [CrossRef]
- Foo, J.B.; Looi, Q.H.; How, C.W.; Lee, S.H.; Al-Masawa, M.E.; Chong, P.P.; Law, J.X. Mesenchymal Stem Cell-Derived Exosomes and MicroRNAs in Cartilage Regeneration: Biogenesis, Efficacy, miRNA Enrichment and Delivery. Pharmaceuticals 2021, 14, 1093. [Google Scholar] [CrossRef]
- Nasirishargh, A.; Kumar, P.; Ramasubramanian, L.; Clark, K.; Hao, D.; Lazar, S.V.; Wang, A. Exosomal microRNAs from mesenchymal stem/stromal cells: Biology and applications in neuroprotection. World J. Stem Cells 2021, 13, 776–794. [Google Scholar] [CrossRef]
- Oveili, E.; Vafaei, S.; Bazavar, H.; Eslami, Y.; Mamaghanizadeh, E.; Yasamineh, S.; Gholizadeh, O. The potential use of mesenchymal stem cells-derived exosomes as microRNAs delivery systems in different diseases. Cell Commun. Signal. CCS 2023, 21, 20. [Google Scholar] [CrossRef]
- Jaukovic, A.; Kukolj, T.; Obradovic, H.; Okic-Dordevic, I.; Mojsilovic, S.; Bugarski, D. Inflammatory niche: Mesenchymal stromal cell priming by soluble mediators. World J. Stem Cells 2020, 12, 922–937. [Google Scholar] [CrossRef]
- Noronha, N.C.; Mizukami, A.; Caliari-Oliveira, C.; Cominal, J.G.; Rocha, J.L.M.; Covas, D.T.; Swiech, K.; Malmegrim, K.C.R. Correction to: Priming approaches to improve the efficacy of mesenchymal stromal cell-based therapies. Stem Cell Res. Ther. 2019, 10, 132. [Google Scholar] [CrossRef] [Green Version]
- Zheng, T.; Li, Y.; Zhang, X.; Xu, J.; Luo, M. Exosomes Derived From miR-212-5p Overexpressed Human Synovial Mesenchymal Stem Cells Suppress Chondrocyte Degeneration and Inflammation by Targeting ELF3. Front. Bioeng. Biotechnol. 2022, 10, 816209. [Google Scholar] [CrossRef]
- Zhou, X.; Luo, D.; Sun, H.; Qi, Y.; Xu, W.; Jin, X.; Li, C.; Lin, Z.; Li, G. MiR-132-3p regulates ADAMTS-5 expression and promotes chondrogenic differentiation of rat mesenchymal stem cells. J. Cell. Biochem. 2018, 119, 2579–2587. [Google Scholar] [CrossRef] [PubMed]
- Katakowski, M.; Buller, B.; Zheng, X.; Lu, Y.; Rogers, T.; Osobamiro, O.; Shu, W.; Jiang, F.; Chopp, M. Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Lett. 2013, 335, 201–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Z.; Tang, X.; Li, Q.; Wang, H.; Sun, H.; Tian, J. Mesenchymal stem cell extracellular vesicles-derived microRNA-194-5p delays the development of intervertebral disc degeneration by targeting TRAF6. Regen. Ther. 2022, 19, 88–96. [Google Scholar] [CrossRef]
- Wu, Y.; Peng, W.; Fang, M.; Wu, M.; Wu, M. MSCs-Derived Extracellular Vesicles Carrying miR-212-5p Alleviate Myocardial Infarction-Induced Cardiac Fibrosis via NLRC5/VEGF/TGF-beta1/SMAD Axis. J. Cardiovasc. Transl. Res. 2022, 15, 302–316. [Google Scholar] [CrossRef]
- Xing, X.; Li, Z.; Yang, X.; Li, M.; Liu, C.; Pang, Y.; Zhang, L.; Li, X.; Liu, G.; Xiao, Y. Adipose-derived mesenchymal stem cells-derived exosome-mediated microRNA-342-5p protects endothelial cells against atherosclerosis. Aging 2020, 12, 3880–3898. [Google Scholar] [CrossRef]
- Liu, W.; Hu, C.; Zhang, B.; Li, M.; Deng, F.; Zhao, S. Exosomal microRNA-342-5p secreted from adipose-derived mesenchymal stem cells mitigates acute kidney injury in sepsis mice by inhibiting TLR9. Biol. Proced. Online 2023, 25, 10. [Google Scholar] [CrossRef]
- Ma, T.; Chen, Y.; Chen, Y.; Meng, Q.; Sun, J.; Shao, L.; Yu, Y.; Huang, H.; Hu, Y.; Yang, Z.; et al. MicroRNA-132, Delivered by Mesenchymal Stem Cell-Derived Exosomes, Promote Angiogenesis in Myocardial Infarction. Stem Cells Int. 2018, 2018, 3290372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, X.; Wang, Y.; Shi, Y.; Li, S.; Liu, J.; Li, X.; Zhong, W.; Pan, Q. Exosomal miR-132-3p from mesenchymal stromal cells improves synaptic dysfunction and cognitive decline in vascular dementia. Stem Cell Res. Ther. 2022, 13, 315. [Google Scholar] [CrossRef]
- Ge, L.; Wang, K.; Lin, H.; Tao, E.; Xia, W.; Wang, F.; Mao, C.; Feng, Y. Engineered exosomes derived from miR-132-overexpresssing adipose stem cells promoted diabetic wound healing and skin reconstruction. Front. Bioeng. Biotechnol. 2023, 11, 1129538. [Google Scholar] [CrossRef]
- Shen, H.; Yao, X.; Li, H.; Li, X.; Zhang, T.; Sun, Q.; Ji, C.; Chen, G. Role of Exosomes Derived from miR-133b Modified MSCs in an Experimental Rat Model of Intracerebral Hemorrhage. J. Mol. Neurosci. MN 2018, 64, 421–430. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Z.; Xing, H.; Wang, Y.; Guo, Y. Exosomes derived from miR-188-3p-modified adipose-derived mesenchymal stem cells protect Parkinson’s disease. Mol. Ther. Nucleic Acids 2021, 23, 1334–1344. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Lv, D.; Song, T.; Niu, C.; Wang, Y. Tumor suppressive role of microRNA-139-5p in bone marrow mesenchymal stem cells-derived extracellular vesicles in bladder cancer through regulation of the KIF3A/p21 axis. Cell Death Dis. 2022, 13, 599. [Google Scholar] [CrossRef] [PubMed]
- Hong, P.; Yu, M.; Tian, W. Diverse RNAs in adipose-derived extracellular vesicles and their therapeutic potential. Mol. Ther. Nucleic Acids 2021, 26, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.A.; Ludwig, R.G.; Garcia-Martin, R.; Brandao, B.B.; Kahn, C.R. Extracellular miRNAs: From Biomarkers to Mediators of Physiology and Disease. Cell Metab. 2019, 30, 656–673. [Google Scholar] [CrossRef] [PubMed]





| 3D MSCs | 3D MSC EXOs | γ-MSCs | γ-MSCs EXOs | ||||
|---|---|---|---|---|---|---|---|
| GO Terms | miRNAs | GO Terms | miRNAs | GO Terms | miRNAs | GO Terms | miRNAs |
| response to hypoxia | miR-100-5p; miR-101-3p; miR-132-3p; miR-144-3p; miR-148a-3p; miR-181c-5p; miR-186-5p; miR-199a-5p; miR-212-3p; miR-23b-3p; miR-296-3p; miR-429 | blood vessel development | let-7a-5p; let-7c-5p; miR-132-3p; miR-200c-3p; miR-212-3p; miR-214-3p; miR-21-5p; miR-22-3p; miR-34a-5p | immune system process | miR-492 | apoptotic signaling pathway | miR-133b; miR-135a-5p; miR-141-3p; miR-486-3p; miR-139-5p; miR-182-5p; miR-133a-3p; miR-429; miR-448; miR-205-5p; miR-198; let-7b-5p; miR-125b-5p |
| response to growth factor | miR-101-3p; miR-124-3p; miR-132-3p; miR-1-3p; miR-144-3p; miR-148a-3p; miR-181c-5p; miR-183-5p; miR-186-5p; miR-194-5p; miR-199a-5p; miR-23b-3p; miR-429; miR-497-5p | hepatic Immune response | let-7a-5p; let-7c-5p | positive regulation of programmed cell death | miR-133b | morphogenesis of a branching epithelium | miR-33b-5p; miR-133b; miR-429; miR-448; miR-205-5p; miR-198; miR-125b-5p |
| cellular response to cytokine stimulus | miR-101-3p; miR-124-3p; miR-132-3p; miR-1-3p; miR-144-3p; miR-148a-3p; miR-183-5p; miR-186-5p; miR-194-5p; miR-199a-5p; miR-212-3p; miR-429; miR-892b | tube morphogenesis | let-7a-5p; miR-130b-3p; miR-132-3p; miR-200c-3p; miR-212-3p; miR-21-5p; miR-22-3p; miR-33b-5p; miR-34a-5p | intrinsic apoptotic signaling pathway | miR-188-3p | chronic inflammatory response | miR-452-5p; miR-125b-5p |
| sprouting angiogenesis | miR-101-3p; miR-124-3p; miR-132-3p; miR-144-3p | vasculature development | let-7a-5p; let-7c-5p; miR-200c-3p; miR-212-3p; miR-214-3p; miR-21-5p; miR-22-3p; miR-34a-5p | lymphocyte activation | miR-139-5p | positive regulation of response to oxidative stress | miR-452-5p; miR-125b-5p |
| apoptotic process | miR-100-5p; miR-101-3p; miR-124-3p; miR-1-3p; miR-148a-3p; miR-149-5p; miR-181c-5p; miR-183-5p; miR-193b-3p; miR-199a-5p; miR-212-3p; miR-23b-3p; miR-296-3p; miR-429; miR-497-5p; miR-518c-3p | apoptotic signaling pathway | let-7b-5p; miR-106b-5p; miR-15b-5p; miR-200c-3p; miR-212-3p; miR-214-3p; miR-21-5p; miR-22-3p; miR-34a-5p; miR-744-5p | cell differentiation | miR-1249-3p; miR-125a-5p; miR-125b-5p; miR-133a-3p; miR-133b; miR-135b-5p; miR-145-5p; miR-19a-3p; miR-204-5p; miR-20a-5p; miR-210-3p; miR-224-5p; miR-27b-3p; miR-331-3p; miR-335-5p; miR-33a-5p; miR-378a-3p; miR-495-3p; miR-518c-3p; miR-9-5p | epithelial to mesenchymal transition | miR-182-5p; miR-429; miR-205-5p; miR-452-5p |
| negative regulation of cell cycle | miR-101-3p; miR-124-3p; miR-144-3p; miR-148a-3p; miR-149-5p; miR-183-5p; miR-186-5p; miR-193b-3p; miR-212-3p; miR-219a-5p; miR-23b-3p; miR-429; miR-615-3p | tube development | miR-130b-3p; miR-132-3p; miR-200c-3p; miR-212-3p; miR-21-5p; miR-22-3p; miR-33b-5p; miR-34a-5p | regulation of phosphatidylinositol 3-kinase signaling | miR-125a-5p; miR-125b-5p; miR-133b; miR-145-5p | activation of MAPK activity | miR-205-5p; miR-125b-5p |
| activation of NF-kappaB-inducing kinase activity | miR-875-5p; miR-892b | positive regulation of immune system process | miR-132-3p; miR-200c-3p; miR-214-3p; miR-21-5p; miR-22-3p; miR-33b-5p; miR-34a-5p; miR-34b-5p | cellular response to growth factor stimulus | miR-125a-5p; miR-125b-5p; miR-133a-3p; miR-133b; miR-135b-5p; miR-145-5p; miR-15a-5p; miR-194-5p; miR-195-5p; miR-19a-3p; miR-204-5p; miR-23b-3p; miR-25-3p; miR-27b-3p; miR-378a-3p; miR-424-5p; miR-495-3p; miR-9-5p | T-helper cell differentiation | miR-33b-5p; miR-9-5p |
| positive regulation of T cell cytokine production | miR-875-5p; miR-892b | regulation of fibroblast proliferation | miR-130b-3p; miR-132-3p; miR-200c-3p; miR-212-3p; miR-21-5p; miR-22-3p; miR-34a-5p; miR-34b-5p | positive regulation of cell growth | miR-125a-5p; miR-125b-5p; miR-133a-3p; miR-133b; miR-145-5p; miR-224-5p | T cell differentiation involved in immune response | miR-33b-5p; miR-9-5p |
| tube development | miR-101-3p; miR-124-3p; miR-132-3p; miR-1-3p; miR-144-3p; miR-148a-3p; miR-199a-5p; miR-212-3p; miR-219a-5p; miR-335-5p; miR-429 | activation of immune response | miR-132-3p; miR-15b-5p; miR-200c-3p; miR-21-5p | response to wounding | miR-125a-5p; miR-133a-3p; miR-133b; miR-145-5p; miR-194-5p; miR-195-5p; miR-19a-3p; miR-204-5p; miR-224-5p; miR-494-3p; miR-9-5p | coronary vasculature development | miR-9-5p; miR-503-5p; let-7c-5p |
| regulation of cell migration involved in sprouting angiogenesis | miR-101-3p; miR-124-3p; miR-132-3p; miR-1-3p; miR-144-3p; miR-497-5p | activation of innate immune response | miR-130b-3p; miR-132-3p; miR-200c-3p; miR-21-5p | anatomical structure morphogenesis | miR-125a-5p; miR-125b-5p; miR-133a-3p; miR-133b; miR-135b-5p; miR-145-5p; miR-195-5p; miR-19a-3p; miR-204-5p; miR-210-3p; miR-224-5p; miR-27b-3p; miR-331-3p; miR-335-5p; miR-33a-5p; miR-424-5p; miR-452-5p; miR-518c-3p; miR-9-5p | anatomical structure morphogenesis | miR-33b-5p; miR-9-5p; miR-1-3p; miR-133b; miR-135a-5p; miR-141-3p; miR-582-5p; miR-182-5p; miR-183-5p; miR-133a-3p; miR-429; miR-448; miR-205-5p; miR-452-5p; miR-125b-5p |
| programmed cell death | miR-100-5p; miR-101-3p; miR-124-3p; miR-1-3p; miR-148a-3p; miR-149-5p; miR-181c-5p; miR-183-5p; miR-193b-3p; miR-199a-5p; miR-212-3p; miR-296-3p; miR-429; miR-497-5p; miR-518c-3p | innate immune response-activating signal transduction | miR-130b-3p; miR-132-3p; miR-200c-3p; miR-21-5p | tube morphogenesis | miR-125a-5p; miR-125b-5p; miR-133a-3p; miR-133b; miR-135b-5p; miR-145-5p; miR-19a-3p; miR-204-5p; miR-210-3p; miR-224-5p; miR-27b-3p; miR-335-5p; miR-452-5p; miR-9-5p | cytokine-mediated signaling pathway | miR-9-5p; miR-1-3p; miR-98-5p; miR-133b; miR-135a-5p; miR-139-5p; miR-183-5p; miR-133a-3p; miR-429; miR-448; miR-205-5p; miR-452-5p; miR-125b-5p |
| tissue morphogenesis | miR-101-3p; miR-124-3p; miR-1-3p; miR-144-3p; miR-148a-3p; miR-186-5p; miR-199a-5p; miR-219a-5p; miR-410-3p; miR-429 | regulation of innate immune response | miR-130b-3p; miR-132-3p; miR-200c-3p; miR-21-5p | cell activation involved in immune response | miR-125b-5p; miR-133a-3p; miR-133b | B cell homeostasis | miR-429; miR-125b-5p |
| negative regulation of cell motility | miR-101-3p; miR-124-3p; miR-1-3p; miR-144-3p; miR-148a-3p; miR-429 | response to growth factor | let-7c-5p; miR-130b-3p; miR-132-3p; miR-15b-5p; miR-200c-3p; miR-214-3p; miR-21-5p; miR-22-3p; miR-33b-5p; miR-34a-5p; miR-34b-5p; miR-424-5p; miR-744-5p | cell differentiation involved in embryonic placenta development | miR-125a-5p; miR-125b-5p; miR-133b | lymphocyte homeostasis | miR-429; miR-125b-5p |
| negative regulation of cellular component movement | miR-101-3p; miR-124-3p; miR-1-3p; miR-144-3p; miR-148a-3p; miR-429 | response to cytokine | miR-132-3p; miR-15b-5p; miR-200c-3p; miR-212-3p; miR-214-3p; miR-21-5p; miR-22-3p; miR-33b-5p; miR-34a-5p; miR-34b-5p; miR-744-5p | leukocyte activation involved in immune response | miR-125b-5p; miR-133a-3p; miR-133b | monocyte differentiation | miR-429; miR-205-5p |
| positive regulation of epithelial cell migration | miR-101-3p; miR-124-3p; miR-132-3p; miR-1-3p; miR-144-3p; miR-149-5p; miR-199a-5p; miR-429 | insulin receptor signaling pathway | miR-15b-5p; miR-200c-3p; miR-21-5p; miR-22-3p | leukocyte mediated immunity | miR-10a-5p; miR-133a-3p; miR-133b | chemokine C-C motif ligand 2 secretion | miR-9-5p; miR-1-3p |
| positive regulation of cell migration involved in sprouting angiogenesis | miR-101-3p; miR-124-3p; miR-132-3p; miR-144-3p; miR-199a-5p | regulation of endothelial cell migration | miR-132-3p; miR-200c-3p; miR-21-5p; miR-22-3p | cytokine-mediated signaling pathway | miR-125a-5p; miR-125b-5p; miR-133a-3p; miR-133b; miR-135b-5p; miR-145-5p; miR-15a-5p; miR-204-5p; miR-224-5p; miR-23b-3p; miR-378a-3p; miR-452-5p; miR-9-5p | coronary vasculature morphogenesis | miR-9-5p; miR-1-3p |
| cellular response to fibroblast growth factor stimulus | miR-124-3p; miR-132-3p; miR-1-3p | negative regulation of apoptotic process | let-7a-5p; let-7c-5p; miR-15b-5p; miR-200c-3p; miR-212-3p; miR-214-3p; miR-21-5p; miR-22-3p; miR-30e-3p; miR-33b-5p; miR-34a-5p; miR-543; miR-744-5p | regulation of apoptotic process | miR-106b-5p; miR-125a-5p; miR-125b-5p; miR-133a-3p; miR-133b; miR-145-5p; miR-15a-5p; miR-195-5p; miR-19a-3p; miR-204-5p; miR-20a-5p; miR-210-3p; miR-27b-3p; miR-331-3p; miR-33a-5p; miR-378a-3p; miR-424-5p; miR-495-3p; miR-518c-3p; miR-9-5p | developmental cell growth | miR-9-5p; miR-1-3p |
| anatomical structure formation involved in morphogenesis | miR-101-3p; miR-124-3p; miR-1-3p; miR-144-3p; miR-148a-3p; miR-199a-5p; miR-29b-1-5p; miR-335-5p; miR-429 | cellular response to cytokine stimulus | miR-132-3p; miR-15b-5p; miR-200c-3p; miR-212-3p; miR-214-3p; miR-21-5p; miR-22-3p; miR-33b-5p; miR-34a-5p; miR-744-5p | positive regulation of cell differentiation | miR-125a-5p; miR-125b-5p; miR-133b; miR-135b-5p; miR-145-5p; miR-19a-3p; miR-204-5p; miR-224-5p; miR-23b-3p; miR-329-3p; miR-518c-3p; miR-9-5p | fibroblast migration | miR-33b-5p; miR-1-3p |
| mesenchyme development | miR-101-3p; miR-124-3p; miR-144-3p; miR-148a-3p; miR-199a-5p; miR-219a-5p; miR-29b-1-5p; miR-410-3p; miR-429 | cellular response to oxygen levels | miR-106b-5p; miR-132-3p; miR-200c-3p; miR-212-3p; miR-214-3p; miR-21-5p; miR-22-3p; miR-33b-5p; miR-34a-5p; miR-543 | positive regulation of endothelial cell proliferation | miR-125a-5p; miR-133b; miR-145-5p | positive regulation of MAP kinase activity | miR-503-5p; miR-429; miR-205-5p; miR-125b-5p |
| fibroblast migration | miR-101-3p; miR-1-3p; miR-144-3p | lymphocyte activation | miR-15b-5p; miR-200c-3p; miR-214-3p; miR-21-5p; miR-22-3p; miR-34a-5p; miR-34b-5p | positive regulation of vascular endothelial cell proliferation | miR-125a-5p; miR-133b; miR-145-5p | leukocyte differentiation | miR-9-5p; miR-133b; miR-182-5p; miR-133a-3p; miR-429; miR-205-5p; miR-125b-5p |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bulati, M.; Gallo, A.; Zito, G.; Busà, R.; Iannolo, G.; Cuscino, N.; Castelbuono, S.; Carcione, C.; Centi, C.; Martucci, G.; et al. 3D Culture and Interferon-γ Priming Modulates Characteristics of Mesenchymal Stromal/Stem Cells by Modifying the Expression of Both Intracellular and Exosomal microRNAs. Biology 2023, 12, 1063. https://doi.org/10.3390/biology12081063
Bulati M, Gallo A, Zito G, Busà R, Iannolo G, Cuscino N, Castelbuono S, Carcione C, Centi C, Martucci G, et al. 3D Culture and Interferon-γ Priming Modulates Characteristics of Mesenchymal Stromal/Stem Cells by Modifying the Expression of Both Intracellular and Exosomal microRNAs. Biology. 2023; 12(8):1063. https://doi.org/10.3390/biology12081063
Chicago/Turabian StyleBulati, Matteo, Alessia Gallo, Giovanni Zito, Rosalia Busà, Gioacchin Iannolo, Nicola Cuscino, Salvatore Castelbuono, Claudia Carcione, Claudio Centi, Gennaro Martucci, and et al. 2023. "3D Culture and Interferon-γ Priming Modulates Characteristics of Mesenchymal Stromal/Stem Cells by Modifying the Expression of Both Intracellular and Exosomal microRNAs" Biology 12, no. 8: 1063. https://doi.org/10.3390/biology12081063
APA StyleBulati, M., Gallo, A., Zito, G., Busà, R., Iannolo, G., Cuscino, N., Castelbuono, S., Carcione, C., Centi, C., Martucci, G., Bertani, A., Baiamonte, M. P., Chinnici, C. M., Conaldi, P. G., & Miceli, V. (2023). 3D Culture and Interferon-γ Priming Modulates Characteristics of Mesenchymal Stromal/Stem Cells by Modifying the Expression of Both Intracellular and Exosomal microRNAs. Biology, 12(8), 1063. https://doi.org/10.3390/biology12081063