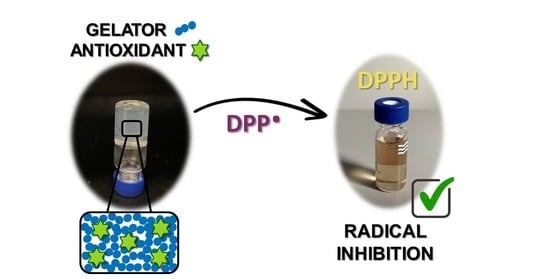
Low-Molecular-Weight Gels as Smart Materials for the Enhancement of Antioxidants Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Chemicals
2.1.2. Postbiotics
2.2. Methods
2.2.1. Solutions of Tested Substances
2.2.2. Synthesis and Characterization of Boc-L-DOPA(Bn)2-OH
2.2.3. Preparation of Gels with α-Tocopherol
2.2.4. Preparation of Gels with Postbiotics
2.2.5. Optical Microscopy
2.2.6. Rheological Properties
2.2.7. Determination of DPPH Radical Scavenging Activity (in Gel/in Solution)
2.2.8. Statistical Analysis
3. Results and Discussion
3.1. LMW Gels with α-Tocopherol
3.2. LMW Gel with Postbiotics (PB)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Draper, E.R.; Adams, D.J. Low-Molecular-Weight Gels: The State of the Art. Chem 2017, 3, 390–410. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Liu, K.; Xing, R.; Yan, X. Peptide self-assembly: Thermodynamics and kinetics. Chem. Soc. Rev. 2016, 45, 5589–5604. [Google Scholar] [CrossRef]
- Hanabusa, K.; Suzuki, M. Development of low-molecular-weight gelators and polymer-based gelators. Polym. J. 2014, 46, 776–782. [Google Scholar] [CrossRef]
- Dastidar, P.; Roy, R.; Parveen, R.; Sarkar, K. Supramolecular Synthon Approach in Designing Molecular Gels for Advanced Therapeutics. Adv. Ther. 2019, 2, 1800061. [Google Scholar] [CrossRef] [Green Version]
- Nicastro, G.; Black, L.M.; Ravarino, P.; Agostino, S.; Faccio, D.; Tomasini, C.; Giuri, D. Controlled Hydrolysis of Odorants Schiff Bases in Low-Molecular-Weight Gels. Int. J. Mol. Sci. 2022, 23, 3105. [Google Scholar] [CrossRef]
- Du, H.; Liu, J.; Pan, B.; Yang, H.-Y.; Liu, G.-B.; Lu, K. Fabrication of the low molecular weight peptide-based hydrogels and analysis of gelation behaviors. Food Hydrocoll. 2022, 131, 107751. [Google Scholar] [CrossRef]
- Oliveira, C.B.P.; Gomes, V.; Ferreira, P.M.T.; Martins, J.A.; Jervis, P.J. Peptide-Based Supramolecular Hydrogels as Drug Delivery Agents: Recent Advances. Gels 2022, 8, 706. [Google Scholar] [CrossRef]
- Okesola, B.O.; Smith, D.K. Applying low-molecular weight supramolecular gelators in an environmental setting-self-assembled gels as smart materials for pollutant removal. Chem. Soc. Rev. 2016, 45, 4226–4251. [Google Scholar] [CrossRef] [Green Version]
- Giuri, D.; D’Agostino, S.; Ravarino, P.; Faccio, D.; Falini, G.; Tomasini, C. Water Remediation from Pollutants Agents by the Use of an Environmentally Friendly Supramolecular Hydrogel. ChemNanoMat 2022, 8, e202200093. [Google Scholar] [CrossRef]
- Giuri, D.; Zanna, N.; Tomasini, C. Low Molecular Weight Gelators Based on Functionalized l-Dopa Promote Organogels Formation. Gels 2019, 5, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guidetti, G.; Giuri, D.; Zanna, N.; Calvaresi, M.; Montalti, M.; Tomasini, C. Biocompatible and Light-Penetrating Hydrogels for Water Decontamination. ACS Omega 2018, 3, 8122–8128. [Google Scholar] [CrossRef]
- Skilling, K.J.; Citossi, F.; Bradshaw, T.D.; Ashford, M.; Kellam, B.; Marlow, M. Insights into low molecular mass organic gelators: A focus on drug delivery and tissue engineering applications. Soft Matter 2014, 10, 237–256. [Google Scholar] [CrossRef]
- Jeong, S.; Lee, S.; Oh, I. Development of Antioxidant-Fortified Oleogel and Its Application as a Solid Fat Replacer to Muffin. Foods 2021, 10, 3059. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhao, Y.; Lu, Z.; Tang, Y. Amino Acid-Modified Conjugated Oligomer Self-Assembly Hydrogel for Efficient Capture and Specific Killing of Antibiotic-Resistant Bacteria. ACS Appl. Mater. Interfaces 2019, 11, 16320–16327. [Google Scholar] [CrossRef]
- Hadian, M.; Rajaei, A.; Mohsenifar, A.; Tabatabaei, M. Encapsulation of Rosmarinus officinalis essential oils in chitosan-benzoic acid nanogel with enhanced antibacterial activity in beef cutlet against Salmonella typhimurium during refrigerated storage. Lwt 2017, 84, 394–401. [Google Scholar] [CrossRef]
- Barreira, J.C.M.; Rodrigues, S.; Carvalho, A.M.; Ferreira, I.C.F.R. Development of hydrosoluble gels with Crataegus monogyna extracts for topical application: Evaluation of antioxidant activity of the final formulations. Ind. Crops Prod. 2013, 42, 175–180. [Google Scholar] [CrossRef]
- Maqsoudlou, A.; Assadpour, E.; Mohebodini, H.; Jafari, S.M. Improving the efficiency of natural antioxidant compounds via different nanocarriers. Adv. Colloid Interface Sci. 2020, 278, 102122. [Google Scholar] [CrossRef] [PubMed]
- Villalva, M.; Jaime, L.; Arranz, E.; Zhao, Z.; Corredig, M.; Reglero, G.; Santoyo, S. Nanoemulsions and acidified milk gels as a strategy for improving stability and antioxidant activity of yarrow phenolic compounds after gastrointestinal digestion. Food Res. Int. 2020, 130, 108922. [Google Scholar] [CrossRef]
- Lee, S.H.; Chow, P.S.; Yagnik, C.K. Developing Eco-Friendly Skin Care Formulations with Microemulsions of Essential Oil. Cosmetics 2022, 9, 30. [Google Scholar] [CrossRef]
- Salazar-Bautista, S.C.; Chebil, A.; Pickaert, G.; Gaucher, C.; Jamart-Gregoire, B.; Durand, A.; Leonard, M. Encapsulation and release of hydrophobic molecules from particles of gelled triglyceride with aminoacid-based low-molecular weight gelators. Colloids Surfaces A Physicochem. Eng. Asp. 2017, 514, 11–20. [Google Scholar] [CrossRef]
- Wei, Q.; Duan, J.; Ma, G.; Zhang, W.; Wang, Q.; Hu, Z. Enzymatic crosslinking to fabricate antioxidant peptide-based supramolecular hydrogel for improving cutaneous wound healing. J. Mater. Chem. B 2019, 7, 2220–2225. [Google Scholar] [CrossRef]
- Butkeviciute, A.; Ramanauskiene, K.; Janulis, V. Formulation of Gels and Emulgels with Malus domestica Borkh: Apple Extracts and Their Biopharmaceutical Evaluation In Vitro. Antioxidants 2022, 11, 373. [Google Scholar] [CrossRef]
- Lutchmanen Kolanthan, V.; Brown, A.; Soobramaney, V.; Philibert, E.G.; Francois Newton, V.; Hosenally, M.; Sokeechand, B.N.; Petkar, G.; Moga, A.; Andres, P.; et al. Clinical Evaluation of Indian Sandalwood Oil and Its Protective Effect on the Skin against the Detrimental Effect of Exposome. Cosmetics 2022, 9, 35. [Google Scholar] [CrossRef]
- Wortzman, M.; Nelson, D.B. A comprehensive topical antioxidant inhibits oxidative stress induced by blue light exposure and cigarette smoke in human skin tissue. J. Cosmet. Dermatol. 2021, 20, 1160–1165. [Google Scholar] [CrossRef]
- Shahzad, A.; Hussain, S.; Anwar, N.; Karim, A.; Aeman, U.; Iqbal, M.J. An overview of free Radicals & antioxidants and its Deletenous actions. Front. Chem. Sci. 2021, 2, 147–164. [Google Scholar] [CrossRef]
- Temova Rakuša, Ž.; Roškar, R. Quality Control of Vitamins A and E and Coenzyme Q10 in Commercial Anti-Ageing Cosmetic Products. Cosmetics 2021, 8, 61. [Google Scholar] [CrossRef]
- Akhtar, N.; Akhtar, N. Development of stable tocopherol succinate-loaded ethosomes to enhance transdermal permeation: In vitro and in vivo characterizations. J. Cosmet. Dermatol. 2022, 21, 4942–4955. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.N.; Gupta, G.; Sharma, P. A comprehensive review of free radicals, antioxidants and their relationship with human ailments. Crit. Rev. Eukaryot. Gene Expr. 2018, 28, 139–154. [Google Scholar] [CrossRef]
- Chaudhari, A.; Dwivedi, M.K. Chapter 1—The concept of probiotics, prebiotics, postbiotics, synbiotics, nutribiotics, and pharmabiotics. In Probiotics in the Prevention and Management of Human Diseases; Dwivedi, M.K., Amaresan, N., Sankaranarayanan, A., Kemp, E.H., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 1–11. ISBN 978-0-12-823733-5. [Google Scholar]
- Zawistowska-Rojek, A.; Zaręba, T.; Tyski, S. Microbiological Testing of Probiotic Preparations. Int. J. Environ. Res. Public Health 2022, 19, 5701. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Wang, J. Oxidative stress tolerance and antioxidant capacity of lactic acid bacteria as probiotic: A systematic review. Gut Microbes 2020, 12, 1801944. [Google Scholar] [CrossRef]
- Ácsová, A.; Hojerová, J.; Martiniaková, S. Efficacy of postbiotics against free radicals and UV radiation. Chem. Pap. 2022, 76, 2357–2364. [Google Scholar] [CrossRef]
- Giuri, D.; Jurković, L.; Fermani, S.; Kralj, D.; Falini, G.; Tomasini, C. Supramolecular Hydrogels with Properties Tunable by Calcium Ions: A Bio-Inspired Chemical System. ACS Appl. Bio Mater. 2019, 2, 5819–5828. [Google Scholar] [CrossRef] [PubMed]
- Di Filippo, M.F.; Giuri, D.; Marchiori, G.; Maglio, M.; Pagani, S.; Fini, M.; Tomasini, C.; Panzavolta, S. Self-assembling of fibers inside an injectable calcium phosphate bone cement: A feasibility study. Mater. Today Chem. 2022, 24, 100991. [Google Scholar] [CrossRef]
- Gaucher, A.; Dutot, L.; Barbeau, O.; Hamchaoui, W.; Wakselman, M.; Mazaleyrat, J.P. Synthesis of terminally protected (S)-β3-H-DOPA by Arndt-Eistert homologation: An approach to crowned β-peptides. Tetrahedron Asymmetry 2005, 16, 857–864. [Google Scholar] [CrossRef]
- Tangpromphan, P.; Duangsrisai, S.; Jaree, A. Development of separation method for Alpha-Tocopherol and Gamma-Oryzanol extracted from rice bran oil using Three-Zone simulated moving bed process. Sep. Purif. Technol. 2021, 272, 118930. [Google Scholar] [CrossRef]
- Patel, A.R.; Remijn, C.; Heussen, P.C.M.; Den Adel, R.; Velikov, K.P. Novel low-molecular-weight-gelator-based microcapsules with controllable morphology and temperature responsiveness. ChemPhysChem 2013, 14, 305–310. [Google Scholar] [CrossRef]
- Sadeer, N.B.; Montesano, D.; Albrizio, S.; Zengin, G.; Mahomoodally, M.F. The versatility of antioxidant assays in food science and safety—Chemistry, applications, strengths, and limitations. Antioxidants 2020, 9, 709. [Google Scholar] [CrossRef] [PubMed]
- Nikiforidis, C.V.; Scholten, E. Self-assemblies of lecithin and α-tocopherol as gelators of lipid material. RSC Adv. 2014, 4, 2466–2473. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Zhang, T.; Chang, M.; Lu, M.; Liu, R.; Jin, Q.; Wang, X. Effects of interaction between α-tocopherol, oryzanol, and phytosterol on the antiradical activity against DPPH radical. LWT 2019, 112, 108206. [Google Scholar] [CrossRef]
- Müller, L.; Theile, K.; Böhm, V. In vitro antioxidant activity of tocopherols and tocotrienols and comparison of vitamin E concentration and lipophilic antioxidant capacity in human plasma. Mol. Nutr. Food Res. 2010, 54, 731–742. [Google Scholar] [CrossRef]
- Iwatsuki, M.; Tsuchiya, J.; Komuro, E.; Yamamoto, Y.; Niki, E. Effects of solvents and media on the antioxidant activity of α-tocopherol. Biochim. Biophys. Acta—Gen. Subj. 1994, 1200, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Averina, O.V.; Poluektova, E.U.; Marsova, M.V.; Danilenko, V.N. Biomarkers and Utility of the Antioxidant Potential of Probiotic Lactobacilli and Bifidobacteria as Representatives of the Human Gut Microbiota. Biomedicines 2021, 9, 1340. [Google Scholar] [CrossRef] [PubMed]
- Bourebaba, Y.; Marycz, K.; Mularczyk, M.; Bourebaba, L. Postbiotics as potential new therapeutic agents for metabolic disorders management. Biomed. Pharmacother. 2022, 153, 113138. [Google Scholar] [CrossRef] [PubMed]
- Ahire, J.J.; Jakkamsetty, C.; Kashikar, M.S.; Lakshmi, S.G.; Madempudi, R.S. In Vitro Evaluation of Probiotic Properties of Lactobacillus plantarum UBLP40 Isolated from Traditional Indigenous Fermented Food. Probiotics Antimicrob. Proteins 2021, 13, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.; Hajipour, N.; Hasannezhad, P.; Baghbanzadeh, A.; Aghebati-Maleki, L. Potential in vivo delivery routes of postbiotics. Crit. Rev. Food Sci. Nutr. 2022, 62, 3345–3369. [Google Scholar] [CrossRef]
- Butera, A.; Gallo, S.; Pascadopoli, M.; Taccardi, D.; Scribante, A. Home Oral Care of Periodontal Patients Using Antimicrobial Gel with Postbiotics, Lactoferrin, and Aloe Barbadensis Leaf Juice Powder vs. Conventional Chlorhexidine Gel: A Split-Mouth Randomized Clinical Trial. Antibiotics 2022, 11, 118. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toronyi, A.Á.; Giuri, D.; Martiniakova, S.; Tomasini, C. Low-Molecular-Weight Gels as Smart Materials for the Enhancement of Antioxidants Activity. Cosmetics 2023, 10, 38. https://doi.org/10.3390/cosmetics10020038
Toronyi AÁ, Giuri D, Martiniakova S, Tomasini C. Low-Molecular-Weight Gels as Smart Materials for the Enhancement of Antioxidants Activity. Cosmetics. 2023; 10(2):38. https://doi.org/10.3390/cosmetics10020038
Chicago/Turabian StyleToronyi, Aneta Ácsová, Demetra Giuri, Silvia Martiniakova, and Claudia Tomasini. 2023. "Low-Molecular-Weight Gels as Smart Materials for the Enhancement of Antioxidants Activity" Cosmetics 10, no. 2: 38. https://doi.org/10.3390/cosmetics10020038
APA StyleToronyi, A. Á., Giuri, D., Martiniakova, S., & Tomasini, C. (2023). Low-Molecular-Weight Gels as Smart Materials for the Enhancement of Antioxidants Activity. Cosmetics, 10(2), 38. https://doi.org/10.3390/cosmetics10020038