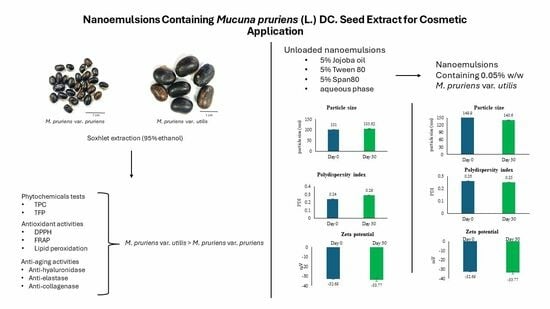
Nanoemulsions Containing Mucuna pruriens (L.) DC. Seed Extract for Cosmetic Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection and Identification of Plant Materials
2.2. Chemicals
2.3. Preparation of M. pruriens Seed Extracts
2.4. Determination of the Total Phenolic Content
2.5. Determination of the Total Flavonoids Content
2.6. Chemical Marker Analysis of M. pruriens Seed Extracts with High-Performance Liquid Chromatography (HPLC)
2.7. Fatty Acids Analysis with Gas Chromatography–Mass Spectrometry (GC–MS)
2.8. Determination of Antioxidant Activity
2.8.1. 1,1-Diphenyl-2-Picrylhydrazyl (DPPH) Radical Scavenging Assay
2.8.2. Ferric-Reducing Antioxidant Power (FRAP) Assay
2.8.3. Lipid Peroxidation Inhibition According to the Ferric Thiocyanate Assay
2.9. Determination of Anti-Aging Activity
2.9.1. Anti-Hyaluronidase Activity According to a Turbidimetric Assay
2.9.2. Anti-Collagenase Activity According to a Spectrophotometric Assay
2.9.3. Anti-Elastase Activity According to Spectrophotometric Assay
2.10. Determination of the Moisturizing Properties with an Occlusion Assay
2.11. Development of Unloaded Nanoemulsions
2.11.1. Preparation of Unloaded Nanoemulsions
2.11.2. Characterization and Stability Testing of Unloaded Nanoemulsions
2.12. Development of Nanoemulsions Containing M. pruriens var. utilis Seeds Extract
2.12.1. Preparation of Nanoemulsions Containing M. pruriens var. utilis Seeds Extract
2.12.2. Characterization and Stability Study of Nanoemulsions Containing M. pruriens var. utilis Seed Extract
2.12.3. Determination of Entrapment Efficiency
2.12.4. Skin Retention Study of Nanoemulsions Containing M. pruriens var. utilis Seed Extract
2.13. Statistical Analysis
3. Results
3.1. M. pruriens Seed Extracts
3.2. Total Phenolic and Total Flavonoid Content of the M. pruriens Seed Extracts
3.3. Chemical Marker Analysis of the M. pruriens Seed Extracts with High-Performance Liquid Chromatography (HPLC)
3.4. Fatty Acids Analysis with Gas Chromatography–Mass Spectrometry (GC–MS)
3.5. Antioxidant Activities of the Mucuna Seeds Extracts
3.5.1. 2,2-diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Assay
3.5.2. Ferric-Reducing Antioxidant Power (FRAP) Assay
3.5.3. Lipid Peroxidation Inhibition According to the Ferric Thiocyanate Assay
3.6. Anti-Aging Activities of Mucuna Seeds Extracts
3.6.1. Anti-Hyaluronidase Activity According to the Turbidimetric Assay
3.6.2. Anti-Collagenase Activity According to the Spectrophotometric Assay
3.6.3. Anti-Elastase Activity by Spectrophotometric Assay
3.7. Moisturizing Properties of Mucuna Seeds Extracts According to the Occlusion Assay
3.8. Development of Unloaded Nanoemulsions
3.8.1. Characterization and Stability of Unloaded Nanoemulsions
3.8.2. Characterization of Nanoemulsions Containing the M. pruriens var. utilis seed extract
3.8.3. Entrapment Efficiency of Nanoemulsions Containing M. pruriens var. utilis Seeds Extract
3.8.4. Skin Retention Study of Nanoemulsions Containing M. pruriens Seeds Extract
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tobin, D.J. Introduction to skin aging. J. Tissue Viability 2017, 26, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Sjerobabski-Masnec, I.; Šitum, M. Skin aging. Acta Clin. Croat. 2010, 49, 515–518. [Google Scholar] [PubMed]
- Fisher, G.J.; Sachs, D.L.; Voorhees, J.J. Ageing: Collagenase-mediated collagen fragmentation as a rejuvenation target. Br. J. Dermatol. 2014, 171, 446–449. [Google Scholar] [CrossRef] [PubMed]
- Heinz, A. Elastases and elastokines: Elastin degradation and its significance in health and disease. Crit. Rev. Biochem. Mol. Biol. 2020, 55, 252–273. [Google Scholar] [CrossRef] [PubMed]
- Hetta, M. Hyaluronidase inhibitors as skin rejuvenating agents from natural source. Int. J. Phytocosmet. Nat. Ingred. 2020, 7, 4. [Google Scholar] [CrossRef]
- Divya, B.J.; Suman, B.; Venkataswamy, M.; ThyagaRaju, K. The traditional uses and pharmacological activities of Mucuna pruriens (L.) DC.: A comprehensive review. Indo. Am. J. Pharm. Res. 2017, 7, 7516–7525. [Google Scholar]
- Ovallath, S.; Deepa, P. The history of parkinsonism: Descriptions in ancient Indian medical literature. Mov. Disord. 2013, 28, 566–568. [Google Scholar] [CrossRef]
- Satheesh, K.D.; Kottai, M.A.; Anton, S.A.; Manavalan, R. In vitro antioxidant activity of various extracts of whole plant of Mucuna pruriens (Linn). Int. J. Pharm. Technol. Res. 2010, 2, 2063–2070. [Google Scholar]
- Rayavarapu, A.K.; Kaladhar, D.S.V.G.K. Evaluation of antimicrobial activity of Mucuna pruriens on plant pathogens. Asian J. Biochem. Pharmaceut. Res. 2011, 2, 593–600. [Google Scholar]
- Bhosale, R.R.; Osmani, R.A.; Ghodake, P.P.; Shaikh, S.M.; Chavan, S.R. Nanoemulsion: A review on novel profusion in advanced drug delivery. Indian J. Pharm. Biol. Res. 2014, 2, 122–127. [Google Scholar] [CrossRef]
- Boonme, P.; Junyaprasert, V.B.; Suksawad, N.; Songkro, S. Microemulsions and nanoemulsions: Novel vehicles for whitening cosmeceuticals. J. Biomed. Nanotech. 2009, 5, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Maneepisamai, Y.; Yasin, P.; Simarugumpai, S. Anti-inflammatory activity and anti-dermatitis-causing bacterial activity of Blumea balsamifera (L.) DC. leaf extracts. Chandra Kasemsarn J. 2012, 18, 33–40. [Google Scholar]
- Garzón, G.A.; Riedl, K.M.; Schwartz, S.J. Determination of anthocyanins, total phenolic content, and antioxidant activity in Andes berry (Rubus glaucus Benth). J. Food Sci. 2009, 74, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Samatha, T.; Shyamsundarachary, R.; Srinivas, P.; Swamy, N.R. Quantification of total phenolic and total flavonoid contents in extracts of Oxylium indicum L. Kurz. Asian J. Pharm. Clin. Res. 2012, 5, 177–179. [Google Scholar]
- Theansungnoen, T.; Nitthikan, N.; Wilai, M.; Chaiwut, P.; Kiattisin, K.; Intharuksa, A. Phytochemical analysis and antioxidant, antimicrobial, and antiaging activities of ethanolic seed extracts of four Mucuna species. Cosmetics 2022, 9, 14. [Google Scholar] [CrossRef]
- Dhanani, T.; Singh, R.; Shah, S.; Kumari, P.; Kumar, S. Comparison of green extraction methods with conventional extraction method for extract yield, L-DOPA concentration and antioxidant activity of Mucuna pruriens seed. Green Chem. Lett. Rev. 2015, 8, 43–48. [Google Scholar] [CrossRef]
- Wan, C.X.; Luo, J.G.; Ren, X.P.; Kong, L.Y. Interconverting flavonostilbenes with antibacterial activity from Sophora alopecuroides. Phytochemistry 2015, 116, 290–297. [Google Scholar] [CrossRef]
- Benziel, F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power” the FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Olszewska, M.A. In vitro antioxidant activity and total phenolic content of the inflorescences, leaves, and fruits of Sorbus torminalis (L.) Crantz. Acta Pol. Pharm. 2011, 68, 945–953. [Google Scholar]
- Be Tu, P.T.; Tawata, S. Antioxidant, anti-aging, and anti-melanogenic properties of the essential oils from two varieties of Alpinia zerumbet. Molecules 2015, 20, 16723–16740. [Google Scholar]
- Thring, T.S.; Hili, P.; Naughton, D.P. Anti-collagenase, anti-elastase, and antioxidant activities of extracts from 21 plants. BMC Complement Altern. Med. 2009, 9, 1–11. [Google Scholar] [CrossRef]
- Kim, Y.J.; Uyama, H.; Kobayashi, S. Inhibition effects of (+)-catechin–aldehyde polycondensates on proteinases causing proteolytic degradation of extracellular matrix. Biochem. Biophys. Res. Commun. 2004, 320, 256–261. [Google Scholar] [CrossRef]
- Da silva, M.J.F.; Rodrigues, A.M.; Vieira, I.R.S.; de Araújo Neves, G.; Menezes, R.R.; do Rosário Gonçalves, E.D.G.; Pires, M.C.C. Development and characterization of a babassu nut oil-based moisturizing cosmetic emulsion with a high sun protection factor. RSC Adv. 2020, 10, 26268–26276. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Rojas-Graü, M.A.; Soliva-Fortuny, R.; Martín-Belloso, O. Effect of processing parameters on physicochemical characteristics of microfluidized lemongrass essential oil-alginate nanoemulsions. Food Hydrocoll. 2013, 30, 401–407. [Google Scholar] [CrossRef]
- Liu, C.H.; Wu, C.T. Optimization of nanostructured lipid carriers for lutein delivery. Colloids Surf. A Physicochem. Eng. 2010, 353, 149–156. [Google Scholar] [CrossRef]
- Sui, X.; Bi, S.; Qi, B.; Wang, Z.; Zhang, M.; Li, Y.; Jiang, L. Impact of ultrasonic treatment on an emulsion system stabilized with soybean protein isolate and lecithin: Its emulsifying property and emulsion stability. Food Hydrocoll. 2017, 63, 727–734. [Google Scholar] [CrossRef]
- Junyaprasert, V.B.; Teeranachaideekul, V.; Souto, E.B.; Boonme, P. Müller RHQ10-loaded NLC versus nanoemulsions: Stability rheology in vitro skin permeation. Int. J. Pharm. 2009, 377, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Misra, L.; Wagner, H. Alkaloidal constituents of Mucuna pruriens seeds. Phytochemistry 2004, 65, 2565–2567. [Google Scholar] [CrossRef]
- Aware, C.B.; Patil, R.R.; Vyavahare, G.D.; Gurme, S.T.; Jadhav, J.P. Ultrasound-assisted aqueous extraction of phenolic, flavonoid compounds and antioxidant activity of Mucuna macrocarpa beans: Response surface methodology optimization. J. Am. Coll. Nutr. 2019, 38, 364–372. [Google Scholar] [CrossRef]
- Njemuva, N.N.; Dickson, N.U.; Elizabeth, A.E.; Uchenna, R.M.; Ogbonnaya, C.N. Evaluation of antioxidant and anti-diabetic effect of Mucuna pruriens extract. Eur. J. Med. Chem. 2019, 27, 1–9. [Google Scholar]
- Jimoh, M.A.; Idris, O.A.; Jimoh, M.O. Cytotoxicity, phytochemical, antiparasitic screening, and antioxidant activities of Mucuna pruriens (Fabaceae). Plants J. 2020, 9, 1249. [Google Scholar] [CrossRef]
- Shay, J.; Elbaz, H.A.; Lee, I.; Zielske, S.P.; Malek, M.H.; Huttemann, M. Molecular mechanisms and therapeutic effects of epicatechin and other polyphenols in cancer, inflammation, diabetes, and neurodegeneration. Oxid. Med. Cell. Longet. 2015, 2015, 181260. [Google Scholar] [CrossRef]
- Kumar, S. The importance of antioxidant and their role in pharmaceutical science—A review. Asian J. Res. Chem. Pharm. Sci. 2014, 1, 27–44. [Google Scholar]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants, and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Gorinstein, S.; Böhm, V.; Schaich, K.M.; Özyürek, M.; Güçlü, K. Methods of measurement and evaluation of natural antioxidant capacity/activity (IUPAC technical report). Pure Appl. Chem. 2013, 85, 957–998. [Google Scholar] [CrossRef]
- Rajeshwar, Y.; Gupta, M.; Mazumder, U.K. Antitumor activity, and in vivo antioxidant status of Mucuna pruriens (Fabaceae) seeds against Ehrlich Ascites carcinoma in swiss albino mice. Iran. J. Pharmacol. Ther. 2005, 4, 46–53. [Google Scholar]
- Draelos, Z.D. The science behind skin care: Moisturizers. J. Cosmet. Dermatol. 2018, 17, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Chantadee, T.; Lertsuphovanit, N.; Phaechamud, T. Application of fatty acid for pharmaceuticals. Thai J. Pharm. Sci. 2019, 14, 1–11. [Google Scholar]
- Ezeagu, I.E.; Gopal, K.A.G.; Khatoon, S. Fatty acid composition of oil from three Mucuna bean varieties from Nigeria—A short report. Pol. J. Food Nutr. Sc. 2005, 55, 151–152. [Google Scholar]
- Kalasariya, H.S.; Patel, N.B.; Yadav, A.; Perveen, K.; Yadav, V.K.; Munshi, F.M.; Yadav, K.K.; Alam, S.; Jung, Y.K.; Jeon, B.H. Characterization of fatty acids, polysaccharides, amino acids, and minerals in marine macroalga chaetomorpha crassa and evaluation of their potentials in skin cosmetics. Molecules 2021, 26, 7515. [Google Scholar] [CrossRef] [PubMed]
- Costa, I.C.; Rodrigues, R.F.; Almeida, F.B.; Favacho, H.A.; Falcão, D.Q.; Ferreira, A.M.; Vilhena, J.C.E.; Florentino, A.C.; Carvalho, J.C.T.; Fernandes, C.P. Development of jojoba oil (Simmondsia chinensis (Link) C.K. Schneid.) based nanoemulsions. Lat. Am. J. Pharm. 2014, 33, 459–463. [Google Scholar]
- Xu, J.; Mukherjee, D.; Chang, S.K. Physicochemical properties, and storage stability of soybean protein nanoemulsions prepared by ultra-high-pressure homogenization. Food Chem. 2018, 240, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Ko, J.A.; Kim, J.T.; Cha, D.S.; Cho, J.H.; Park, H.J.; Shin, G.H. Preparation of a capsaicin loaded nanoemulsion for improving skin penetration. J. Agric. Food Chem. 2014, 62, 725–732. [Google Scholar] [CrossRef] [PubMed]


















| Time (s) | Value (%) | |
|---|---|---|
| Formic Acid | Absolute Methanol | |
| 0.01 | 50 | 50 |
| 1.45 | 2 | 98 |
| 1.75 | 30 | 70 |
| Formulation | Jojoba Oil (% w/w) | Tween® 80 to Span® in Rato of 1:1 (% w/w) | Deionized Water (% w/w) |
|---|---|---|---|
| 1 | 5 | 5 (2.5:2.5) | 90 |
| 2 | 5 | 10 (5:5) | 85 |
| 3 | 5 | 15 (7.5:7.5) | 80 |
| Type | %yield |
|---|---|
| M. pruriens var. utilis | |
| M. pruriens var. pruriens |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chookiat, S.; Theansungnoen, T.; Kiattisin, K.; Intharuksa, A. Nanoemulsions Containing Mucuna pruriens (L.) DC. Seed Extract for Cosmetic Applications. Cosmetics 2024, 11, 29. https://doi.org/10.3390/cosmetics11010029
Chookiat S, Theansungnoen T, Kiattisin K, Intharuksa A. Nanoemulsions Containing Mucuna pruriens (L.) DC. Seed Extract for Cosmetic Applications. Cosmetics. 2024; 11(1):29. https://doi.org/10.3390/cosmetics11010029
Chicago/Turabian StyleChookiat, Suwaporn, Tinnakorn Theansungnoen, Kanokwan Kiattisin, and Aekkhaluck Intharuksa. 2024. "Nanoemulsions Containing Mucuna pruriens (L.) DC. Seed Extract for Cosmetic Applications" Cosmetics 11, no. 1: 29. https://doi.org/10.3390/cosmetics11010029
APA StyleChookiat, S., Theansungnoen, T., Kiattisin, K., & Intharuksa, A. (2024). Nanoemulsions Containing Mucuna pruriens (L.) DC. Seed Extract for Cosmetic Applications. Cosmetics, 11(1), 29. https://doi.org/10.3390/cosmetics11010029