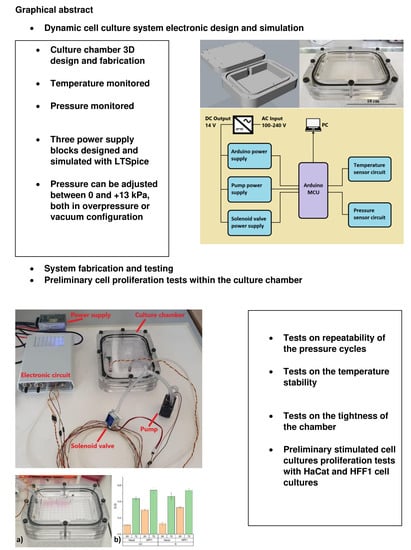
Open-Source Culture Platform for Multi-Cell Type Study with Integrated Pneumatic Stimulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Culture Chamber Design and Fabrication
2.2. Electronic Circuit Design and Fabrication
2.3. Arduino External Power Supply
2.4. Pump Power Supply
2.5. Solenoid Valve Power Supply
2.6. PCB Design and Fabrication
2.7. Arduino Sketch
2.8. Cell Viability Assay
3. Results and Discussion
3.1. LTSpice Simulations
3.2. Tightness of the Culture Chamber
3.3. Stability of the Pressure Cycles
3.4. Temperature Stability
3.5. HaCaT and HFF-1 Proliferation Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bissell, M.J.; Hines, W.C. Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat. Med. 2011, 17, 320–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polacheck, W.J.; Li, R.; Uzel, S.G.M.; Kamm, R.D. Microfluidic platforms for mechanobiology. Lab Chip 2013, 13, 2252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.; Cai, L.-H.; Lan, B.; Fredberg, J.J. Hidden in the mist no more: Physical force in cell biology. Nat. Methods 2016, 13, 124–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levental, K.R.; Yu, H.; Kass, L.; Lakins, J.N.; Egeblad, M.; Erler, J.T.; Fong, S.F.; Csiszar, K.; Giaccia, A.; Weninger, W.; et al. Matrix Crosslinking Forces Tumor Progression by Enhancing Integrin Signaling. Cell 2009, 139, 891–906. [Google Scholar] [CrossRef] [Green Version]
- Takao, S.; Taya, M.; Chiew, C. Mechanical stress-induced cell death in breast cancer cells. Biol. Open 2019, 8, bio.043133. [Google Scholar] [CrossRef] [Green Version]
- Hosmane, S.; Fournier, A.; Wright, R.; Rajbhandari, L.; Siddique, R.; Yang, I.H.; Ramesh, K.T.; Venkatesan, A.; Thakor, N. Valve-based microfluidic compression platform: Single axon injury and regrowth. Lab Chip 2011, 11, 3888. [Google Scholar] [CrossRef]
- Tse, J.M.; Cheng, G.; Tyrrell, J.A.; Wilcox-Adelman, S.A.; Boucher, Y.; Jain, R.K.; Munn, L.L. Mechanical compression drives cancer cells toward invasive phenotype. Proc. Natl. Acad. Sci. USA 2012, 101, 911–916. [Google Scholar] [CrossRef] [Green Version]
- Kalli, M.; Papageorgis, P.; Gkretsi, V.; Stylianopoulos, T. Solid Stress Facilitates Fibroblasts Activation to Promote Pancreatic Cancer Cell Migration. Ann. Biomed. Eng. 2018, 46, 657–669. [Google Scholar] [CrossRef]
- Parmeggiani, M.; Villata, S.; Baruffaldi, D.; Marasso, S.L.; Canavese, G.; Cocuzza, M.; Pirri, C.F.; Frascella, F. A programmable culture platform for hydrostatic stimulation and in situ pH sensing of lung cancer cells with organic electrochemical transistors. Micro Nano Eng. 2022, 16, 100147. [Google Scholar] [CrossRef]
- Lan, B.; Mitchel, J.A.; O’Sullivan, M.J.; Park, C.Y.; Kim, J.H.; Cole, W.C.; Butler, J.P.; Park, J.A. Airway epithelial compression promotes airway smooth muscle proliferation and contraction. Am. J. Physiol. -Lung Cell. Mol. Physiol. 2018, 315, L645–L652. [Google Scholar] [CrossRef]
- Marozkina, N.; Bosch, J.; Cotton, C.; Smith, L.; Seckler, J.; Zaman, K.; Rehman, S.; Periasamy, A.; Gaston, H.; Altawallbeh, G.; et al. Cyclic compression increases F508 Del CFTR expression in ciliated human airway epithelium. Am. J. Physiol. -Lung Cell. Mol. Physiol. 2019, 317, L247–L258. [Google Scholar] [CrossRef] [PubMed]
- Kılıç, A.; Ameli, A.; Park, J.A.; Kho, A.T.; Tantisira, K.; Santolini, M.; Cheng, F.; Mitchel, J.A.; McGill, M.; O’Sullivan, M.J.; et al. Mechanical forces induce an asthma gene signature in healthy airway epithelial cells. Sci Rep. 2020, 10, 966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, M.; Ho, K.K.; Tong, Z.; Deng, L.; Liu, A.P. Compressive Stress Enhances Invasive Phenotype of Cancer Cells via Piezo1 Activation. Cell Biol. 2019. Preprint. [Google Scholar] [CrossRef] [Green Version]
- Novak, C.M.; Horst, E.N.; Lin, E.; Mehta, G. Compressive Stimulation Enhances Ovarian Cancer Proliferation, Invasion, Chemoresistance, and Mechanotransduction via CDC42 in a 3D Bioreactor. Cancers 2020, 12, 1521. [Google Scholar] [CrossRef] [PubMed]
- Asem, M.; Young, A.; Oyama, C.; ClaureDeLaZerda, A.; Liu, Y.; Ravosa, M.; Gupta, V.; Jewell, A.; Khabele, D.; Stack, M.S. Ascites-induced compression alters the peritoneal microenvironment and promotes metastatic success in ovarian cancer. Sci Rep. 2020, 10, 11913. [Google Scholar] [CrossRef] [PubMed]
- Carlos-Oliveira, M.; Lozano-Juan, F.; Occhetta, P.; Visone, R.; Rasponi, M. Current strategies of mechanical stimulation for maturation of cardiac microtissues. Biophys. Rev. 2021, 13, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Lui, C.; Chin, A.F.; Park, S.; Yeung, E.; Kwon, C.; Tomaselli, G.; Chen, Y.; Hibino, N. Mechanical stimulation enhances development of scaffold-free, 3D-printed, engineered heart tissue grafts. J. Tissue Eng. Regen. Med. 2021, 15, 503–512. [Google Scholar] [CrossRef]
- Kim, Y.C.; Kang, J.H.; Park, S.J.; Yoon, E.S.; Park, J.K. Microfluidic biomechanical device for compressive cell stimulation and lysis. Sens. Actuators B Chem. 2007, 128, 108–116. [Google Scholar] [CrossRef]
- Lee, D.; Erickson, A.; You, T.; Dudley, A.T.; Ryu, S. Pneumatic microfluidic cell compression device for high-throughput study of chondrocyte mechanobiology. Lab Chip 2018, 18, 2077–2086. [Google Scholar] [CrossRef]
- Hsieh, H.Y.; Camci-Unal, G.; Huang, T.W.; Liao, R.; Chen, T.J.; Paul, A.; Tseng, F.G.; Khademhosseini, A. Gradient static-strain stimulation in a microfluidic chip for 3D cellular alignment. Lab Chip 2014, 14, 482–493. [Google Scholar] [CrossRef]
- Klymenko, Y.; Wates, R.B.; Weiss-Bilka, H.; Lombard, R.; Liu, Y.; Campbell, L.; Kim, O.; Wagner, D.; Ravosa, M.J.; Stack, M.S. Modeling the effect of ascites-induced compression on ovarian cancer multicellular aggregates. Dis. Model. Mech. 2018, 11, dmm034199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bregenzer, M.E.; Horst, E.N.; Mehta, P.; Novak, C.M.; Repetto, T. The Role of Cancer Stem Cells and Mechanical Forces in Ovarian Cancer Metastasis. Cancers 2019, 11, 1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, R.K.; Martin, J.D.; Stylianopoulos, T. The Role of Mechanical Forces in Tumor Growth and Therapy. Annu. Rev. Biomed. Eng. 2014, 16, 321–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hintz, K.S.; Vedel, H.; Kaas, E. Collecting and processing of barometric data from smartphones for potential use in numerical weather prediction data assimilation. Meteorol. Appl. 2019, 26, 733–746. [Google Scholar] [CrossRef]











| ADJ Resistance | VOUT, Arduino Power Supply (R1) | VOUT, Pump Power Supply (R2) | VOUT, Valve Power Supply (R3) |
|---|---|---|---|
| 1 Ω | 1.3 V | 1.3 V | 7.2 V |
| 200 Ω | 2.49 V | 2.49 V | 8.4 V |
| 400 Ω | 3.7 V | 3.7 V | 9.6 V |
| 600 Ω | 4.7 V | 4.7 V | 10.8 V |
| 800 Ω 1 kΩ | 6.1 V 7.2 V | 6.1 V 7.2 V | 12.0 V 12.5 V |
| Pressure Range | Leakage Rate without T Sensor | Leakage Rate with T Sensor |
|---|---|---|
| 30–18 kPa | −7.2·10−2 Pa/s | −6.8 Pa/s |
| 6.6–4.6 kPa | −2.2·10−2 Pa/s | −7.7·10−1 Pa/s |
| 1.5–0.5 kPa | −1.1·10−2 Pa/s | −8.2·10−2 Pa/s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cacocciola, N.; Marasso, S.L.; Canavese, G.; Cocuzza, M.; Pirri, C.F.; Frascella, F. Open-Source Culture Platform for Multi-Cell Type Study with Integrated Pneumatic Stimulation. Electronics 2023, 12, 73. https://doi.org/10.3390/electronics12010073
Cacocciola N, Marasso SL, Canavese G, Cocuzza M, Pirri CF, Frascella F. Open-Source Culture Platform for Multi-Cell Type Study with Integrated Pneumatic Stimulation. Electronics. 2023; 12(1):73. https://doi.org/10.3390/electronics12010073
Chicago/Turabian StyleCacocciola, Nicolò, Simone Luigi Marasso, Giancarlo Canavese, Matteo Cocuzza, Candido Fabrizio Pirri, and Francesca Frascella. 2023. "Open-Source Culture Platform for Multi-Cell Type Study with Integrated Pneumatic Stimulation" Electronics 12, no. 1: 73. https://doi.org/10.3390/electronics12010073
APA StyleCacocciola, N., Marasso, S. L., Canavese, G., Cocuzza, M., Pirri, C. F., & Frascella, F. (2023). Open-Source Culture Platform for Multi-Cell Type Study with Integrated Pneumatic Stimulation. Electronics, 12(1), 73. https://doi.org/10.3390/electronics12010073