Strategic Syntheses of Vine and Wine Resveratrol Derivatives to Explore Their Effects on Cell Functions and Dysfunctions
Abstract
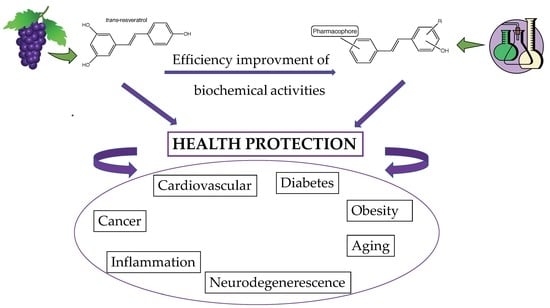
:1. Introduction
2. Which Strategies to Modify trans-Resveratrol
3. Phenyl Rings Substitution of trans-Resveratrol by Hydroxy, Methoxy, and Halogen Groups
4. Phenyl Rings Substitution of trans-Resveratrol by Functionalized Groups or Bioactive Moieties
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hartwig, U.A.; Joseph, C.M.; Phillips, D.A. Flavonoids released naturally from alfalfa seeds enhance growth rate of Rhizobium meliloti. Plant. Physiol. 1991, 95, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Adrian, M.; Jeandet, P. Effects of resveratrol on the ultrastructure of Botrytis cinerea conidia and biological significance in plant/pathogen interactions. Fitoterapia 2012, 83, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- Archetti, M.; Döring, T.F.; Hagen, S.B.; Hughes, N.M.; Leather, S.R.; Lee, D.W.; Lev-Yadun, S.; Manetas, Y.; Ougham, H.J.; Schaberg, P.G.; et al. Unravelling the evolution of autumn colours: An interdisciplinary approach. Trends Ecol. Evol. 2009, 24, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Pirola, L.; Frödjö, S. Resveratrol: One molecule, many targets. IUBMB Life 2008, 60, 323–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mubarak, A.; Swinny, E.E.; Ching, S.Y.L.; Jacob, S.R.; Lacey, K.; Hodgson, J.M.; Croft, K.D.; Considine, M.J. Polyphenol composition of plum selections in relation to total antioxidant capacity. J. Agric. Food Chem. 2012, 60, 10256–10262. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, R.M.; Naidoo, N.; Landberg, R. Dietary flavonoids and the development of type 2 diabetes and cardiovascular diseases. Curr. Opin. Lipidol. 2013, 24, 25–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siemann, E.H.; Creasy, L.L. Concentration of the phytoalexin resveratrol in wine. Am. J. Enol. Vitic. 1992, 43, 49–52. [Google Scholar]
- Chen, H.; Tuck, T.; Ji, X.; Zhou, X.; Kelly, G.; Cuerrier, A.; Zhang, J. Quality assessment of japanese knotweed (Fallopia japonica) grown on Prince Eward Island as a source of resveratrol. J. Agric. Food Chem. 2013, 61, 6383–6392. [Google Scholar] [CrossRef]
- Sobolev, V.S.; Khan, S.I.; Tabanca, N.; Wedge, D.E.; Manly, S.P.; Cutler, S.J.; Coy, M.R.; Becnel, J.J.; Neff, S.A.; Gloer, J.B. Biological activity of peanut (Arachis hypogaea) phytoalexins and selected natural and synthetic stilbenoids. J. Agric. Food Chem. 2011, 59, 1673–1682. [Google Scholar] [CrossRef]
- Adrian, M.; Jeandet, P.; Veneau, J.; Weston, L.A.; Bessis, R. Bological activity od resveratrol, a stilbenic compound from grapevines against Botritys Cinerea the causal agent for gray mold. J. Chem. Ecol. 1997, 23, 1689–1701. [Google Scholar] [CrossRef]
- Gülçin, I. Antioxidant properties of resveratrol: A structure-activity insight. Innov. Food Sci. Emerg. Technol. 2010, 11, 210–218. [Google Scholar] [CrossRef]
- Khan, O.S.; Bhat, A.A.; Krishnankutty, R.; Mohammad, R.M.; Uddin, S. Therapeutic potential of resveratrol in lymphoid malignancies. Nutr. Cancer 2016, 68, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Yiu, C.Y.; Chen, S.Y.; Chang, L.K.; Chiu, Y.F.; Lin, T.P. Inhibitory effects of resveratrol on the Epstein-Barr virus lytic cycle. Molecules 2010, 15, 7115–7124. [Google Scholar] [CrossRef]
- Tili, E.; Michaille, J.J.; Adair, B.; Alder, H.; Limagne, E.; Taccioli, C.; Ferracin, M.; Delmas, D.; Latruffe, N.; Croce, C.M. Resveratrol decreases the levels of miR-155 by upregulating miR-663, a microRNA targeting JunB and JunD. Carcinogenesis 2010, 31, 1561–1566. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K.; Pistell, P.J.; et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaminski, J.; Lançon, A.; Aires, V.; Limagne, E.; Tili, E.; Michaille, J.J.; Latruffe, N. Resveratrol initiates differentiation of mouse skeletal muscle-derived C2C12 myoblasts. Biochem. Pharmacol. 2012, 84, 1251–1259. [Google Scholar] [CrossRef] [PubMed]
- Namsi, A.; Nury, T.; Hamdouni, H.; Yammine, A.; Vejux, A.; Vervandier–Fasseur, D.; Latruffe, N.; Masmoudi-Kouki, O.; Lizard, G. Induction of neuronal differentiation of murine N2a cells by two polyphenols present in the mediterranean diet mimicking neurotrophins activities: Resveratrol and apigenin. Diseases 2018, 6, 67. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Agrawal, M.; Doré, S. Neuroprotective properties and mechanisms of resveratrol in in vitro and in vivo experimental cerebral stroke models. ACS Chem. Neurosci. 2013, 4, 1151–1162. [Google Scholar] [CrossRef] [PubMed]
- Stef, G.; Csiszar, A.; Lerea, K.; Ungvari, Z.; Veress, G. Resveratrol inhibits aggregation of platelets from high-risk cardiac patients with aspirin resistance. J. Cardiovasc. Pharmacol. 2006, 48, 1–5. [Google Scholar] [CrossRef]
- Britton, R.G.; Kovoor, C.; Brown, K. Direct molecular targets of resveratrol: Identifying key interactions to unlock complex mechanisms. Ann. NY Acad. Sci. 2015, 1348, 124–133. [Google Scholar] [CrossRef]
- Sajish, P.; Schimmel, P. A human tRNA synthetase is a potent PARP1-activating effector target for resveratrol. Nature 2015, 519, 370–373. [Google Scholar] [CrossRef] [PubMed]
- Caruso, F.; Tanski, J.; Villegas-Estrada, A.; Rossi, M. Structural basis for antioxidant activity of trans-resveratrol: Ab initio calculations and crystal and molecular structure. J. Agric. Food Chem. 2004, 52, 7279–7285. [Google Scholar] [CrossRef] [PubMed]
- Takaoka, M. Phenolic substances of white hellebore (Veratrum grandiflorum Loes.fil.). J. Faculty Sci. 1940, 3, 1–16. [Google Scholar]
- Chaher, N.; Arraki, K.; Dillisenger, E.; Temsamani, H.; Bernillon, S.; Pedrot, E.; Delaunay, J.C.; Mérillon, J.M.; Monti, J.P.; Izard, J.C.; et al. Bioactive stilbenes from Vitis vinifera grapevine shoots extracts. J. Sci. Food Agric. 2014, 94, 951–954. [Google Scholar] [CrossRef]
- Fan, E.; Zhang, K.; Zhu, M.; Wang, Q. Obtaining resveratrol: From chemical synthesis to biotechnological production. Mini-Rev. Org. Chem. 2010, 7, 272–281. [Google Scholar] [CrossRef]
- Marié, T.; Willig, G.; Teixeira, A.R.S.; Gazaneo Barboza, E.; Kotland, A.; Gratia, A.; Courot, E.; Hubert, J.; Renault, J.H.; Allais, F. Enzymatic synthesis of resveratrol α-glycosides from β-cyclodextrin-resveratrol complex in water. ACS Sustain. Chem. Eng. 2018, 6, 5370–5380. [Google Scholar] [CrossRef]
- Kursvietiene, L.; Staneviciene, I.; Mongirdiene, A.; Bernatoniene, J. Multiplicity of effects and health benefits of resveratrol. Medicina 2016, 52, 148–155. [Google Scholar] [CrossRef]
- Delmas, D.; Aires, V.; Limagne, E.; Dutartre, P.; Mazué, F.; Ghiringhelli, F.; Latruffe, N. Transport, stability and biological activity of resveratrol. Ann. NY Acad. Sci. 2011, 1215, 48–59. [Google Scholar] [CrossRef]
- Giacomini, E.; Rupiani, S.; Guidotti, L.; Recanatini, M.; Roberti, M. The use of stilbene scaffold in medicinal chemistry and Multi Target Drug design. Curr. Med. Chem. 2016, 23, 2439–2489. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Chen, H.; Yao, X.; Xiao, Y.; Zeng, X.; Zheng, Q.; Wei, Y.; Song, C.; Zhang, Y.; Zhu, P.; et al. Synthetic resveratrol derivatives and their biological activities. A review. Open J. Med. Chem. 2015, 5, 97–105. [Google Scholar] [CrossRef]
- Xiao, Y.; Chen, H.; Song, C.; Zeng, X.; Zheng, Q.; Zhang, Y.; Lei, X.; Zheng, X. Pharmacological activities and structure-modification of resveratrol analogues. Pharmazie 2015, 70, 765–771. [Google Scholar] [PubMed]
- Nawaz, W.; Zhou, Z.; Deng, S.; Ma, X.; Ma, X.; Li, C.; Shu, X. Therapeutic versatility of resveratrol derivatives. Nutrients 2017, 9, 1188. [Google Scholar] [CrossRef] [PubMed]
- Biasutto, L.; Mattarei, A.; Azzolini, M.; La Spina, M.; Sassi, N.; Romio, M.; Paradisi, C.; Zoratti, M. Resveratrol derivatives as a pharmacological tool. Ann. NY Acad. Sci. 2017, 1403, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Nicolas, J.M.; Garcia-Carmona, F. Aggregation state and pKa values of (E)-resveratrol as determined by fluorescence spectroscopy and UV-visible absorption. J. Agric. Food Chem. 2008, 56, 7600–7605. [Google Scholar] [CrossRef] [PubMed]
- Cardile, V.; Chillemi, R.; Lombardo, L.; Sciuto, S.; Spatafora, C.; Tringali, C. Antiproliferative activity of methylated analogues of E- and Z-resveratrol. Z. Naturforsch. C 2007, 62, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Peng, B.; Yan, W. Measurement and correlation of solubility of trans-resveratrol in 11 solvents at T = (278.2, 282.2, 298.2, 308.2 and 318.2) K. J. Chem. Thermodyn. 2008, 40, 735–738. [Google Scholar] [CrossRef]
- Ruan, B.F.; Huang, X.F.; Ding, H.; Xu, C.; Ge, H.M.; Zhu, H.L.; Tan, R.X. Synthesis and cytotoxic evaluation of a series of resveratrol derivatives. Chem. Biodiv. 2006, 3, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Solladié, G.; Pasturel-Jacopé, Y.; Maignan, J. A re-investigation of resveratrol synthesis by Perkin reaction. Application to the synthesis of aryl cinnamic acids. Tetrahedron 2003, 59, 3315–3321. [Google Scholar] [CrossRef]
- Chalal, M.; Vervandier-Fasseur, D.; Meunier, P.; Cattey, H.; Hierso, J.C. Synthesis of polyfunctionalized resveratrol derivatives using Wittig and Heck protocols. Tetrahedron 2012, 68, 3899–3907. [Google Scholar] [CrossRef]
- Vervandier-Fasseur, D.; Chalal, M.; Meunier, P. Procédé de préparation du trans-resvératrol et de ses analogues par la réaction de Wittig. French Patent 2011, 11, 56293. [Google Scholar]
- Das, J.; Pany, S.; Majhi, A. Chemical modifications of resveratrol for improved protein kinase C alpha activity. Bioorg. Med. Chem. 2011, 19, 5321–5333. [Google Scholar] [CrossRef] [PubMed]
- Bertini, S.; Calderone, V.; Carboni, I.; Maffei, R.; Martelli, A.; Martinelli, A.; Minutolo, F.; Rajabi, M.; Testai, L.; Tuccinardi, T.; et al. Synthesis of heterocycle-based analogs of resveratrol and their antitumor and vasorelaxing properties. Bioorg. Med. Chem. 2010, 18, 6715–6724. [Google Scholar] [CrossRef] [PubMed]
- Drabikova, K.; Perecko, T.; Nosal, R.; Harmatha, J.; Smidrkal, J.; Jancinova, V. Polyphenol derivatives–Potential regulators of neutrophil activity. Interdiscip. Toxicol. 2012, 5, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Ficarra, S.; Tellone, E.; Pirolli, D.; Russo, A.; Barreca, D.; Galtieri, A.; Giardina, B.; Gavezzotti, P.; Riva, S.; De Rosa, M.C. Insights into the properties of the two enantiomers of trans-δ-viniferin, a resveratrol derivative: Antioxidant activity, biochemical and molecular modeling studies of its interactions with hemoglobin. Mol. Biosyst. 2016, 12, 1276–1286. [Google Scholar] [CrossRef] [PubMed]
- Esatbeyoglu, T.; Ewald, P.; Yasui, Y.; Yokokawa, H.; Wagner, A.E.; Matsugo, S.; Winterhalter, P.; Rimbach, G. Chemical characterization, free radical scavenging, and cellular antioxidant and anti-inflammatory properties of a stilbenoid-rich root extract of Vitis vinifera. Oxid. Med. Cell. Longev. 2015, 2016, 8591286. [Google Scholar] [PubMed]
- Rossi, M.; Caruso, F.; Opazo, C.; Salciccioli, J. Crystal and molecular structure of piceatannol; scavenging features of resveratrol and piceatannol on hydroxyl and peroxyl radicals and docking with transthyretin. J. Agric. Food Chem. 2008, 56, 10557–10566. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Caruso, F.; Antonioletti, R.; Viglianti, A.; Traversi, G.; Leone, S.; Basso, E.; Cozzi, R. Scavenging of hydroxyl radical by resveratrol and related natural stilbenes after hydrogen peroxide attack on DNA. Chem. Biol. Interact. 2013, 206, 175–185. [Google Scholar] [CrossRef]
- Thakkar, K.; Geahlen, R.L.; Cushman, M. Synthesis and protein-tyrosine kinase inhibitory activity of polyhydroxylated stilbene analogues of piceatannol. J. Med. Chem. 1993, 36, 2950–2955. [Google Scholar] [CrossRef]
- Spatafora, C.; Tringali, C. Natural-derived polyphenols as potential anticancer agents. Anti-Cancer Agents Med. Chem. 2012, 12, 902–918. [Google Scholar] [CrossRef]
- Keylor, M.H.; Matsuura, B.S.; Stephenson, C.R.J. Chemistry and biology of resveratrol-derived natural products. Chem. Rev. 2015, 115, 8876–9027. [Google Scholar] [CrossRef]
- Murias, M.; Handler, N.; Erker, T.; Pleban, K.; Ecker, G.; Saiko, P.; Szekeres, T.; Jäger, W. Resveratrol analogues as selective cyclooxygenase-2 inhibitors: Synthesis and structure-activity relationship. Bioorg. Med. Chem. 2004, 12, 5571–5578. [Google Scholar] [CrossRef]
- Murias, M.; Jäger, W.; Handler, N.; Erker, T.; Horvath, Z.; Szekeres, T.; Nohl, H.; Gille, L. Antioxidant, prooxidant and cytotoxic activity of hydroxylated resveratrol analogues: Structure-activity relationship. Biochem. Pharmacol. 2005, 69, 903–912. [Google Scholar] [CrossRef]
- Murias, M.; Luczak, M.W.; Niepsuj, A.; Krajka-Kuzniak, V.; Zielinska-Przyjemska, M.; Jagodzinski, P.P.; Jäger, W.; Szekeres, T.; Jodynis-Liebert, J. Cytotoxic activity of 3,3′,4,4′,5,5′-hexahydroxystilbene against breast cancer cells is mediated by induction of p53 and downregulation of mitochondrial superoxide dismutase. Toxicol. In Vitro 2008, 22, 1361–1370. [Google Scholar] [CrossRef] [PubMed]
- Kucinska, M.; Piotrowska, H.; Luczak, M.W.; Mikula-Pietrasik, J.; Ksiazek, K.; Wozniak, M.; Wierzchowski, M.; Dudka, J.; Jäger, W.; Murias, M. Effects of hydroxylated resveratrol analogs on oxidative stress and cancer cells death in human acute T cell leukemia cell line. Prooxidative potential of hydroxylated resveratrol analogs. Chem. Biol. Interact. 2014, 209, 96–110. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.S.; Quashie, P.K.; Mesplède, T.; Xu, H.; Quan, Y.; Jaeger, W.; Szekeres, T.; Wainberg, M.A. A resveratrol analog termed 3,3′4,4′,5,5′ hexahydroxy-trans-stilbene is a potent HIV-inhibitor. J. Med. Virol. 2015, 87, 2054–2060. [Google Scholar] [CrossRef] [PubMed]
- Gosslau, A.; Chen, M.; Ho, C.T.; Chen, K.Y. A methoxy derivative of resveratrol analogue selectively induced activation of the mitochondrial apoptotic pathway in transformed fibroblasts. Br. J. Cancer 2005, 92, 513–521. [Google Scholar] [CrossRef] [Green Version]
- Androutsopoulos, V.P.; Fragiadaki, I.; Spandidos, D.A.; Tosca, A. The resveratrol analogue, 3,4,5,4′-trans-tetramethoxystilbene, inhibits the growth of A375 melanoma cells through multiple anticancer modes of action. Int. J. Oncol. 2016, 49, 1305–1314. [Google Scholar] [CrossRef] [PubMed]
- Cichocki, M.; Baer-Dubowska, W.; Wierzchowski, M.; Murias, M.; Jodynis-Liebert, J. 3,4,5,4′-trans-tetramethoxystilbene (DMU-212) modulates the activation of NF-κB, AP-1, and STAT3 transcription factors in rat liver carcinogenesis induced by initiation-promotion regimen. Mol. Cell. Biochem. 2014, 391, 27–35. [Google Scholar] [CrossRef]
- Traversi, G.; Fiore, M.; Percario, Z.; Degrassi, F.; Cozzi, R. The resveratrol analogue trimethoxystilbene inhibits cancer cell growth by inducing multipolar cell mitosis. Mol. Carcinogen. 2017, 56, 1117–1126. [Google Scholar] [CrossRef]
- Mazué, F.; Colin, D.; Gobbo, J.; Wegner, M.; Rescifina, A.; Spatafora, C.; Fasseur, D.; Delmas, D.; Meunier, P.; Tringali, C.; Latruffe, N. Structural determinants of resveratrol for cell proliferation inhibition potency; experimental and docking studies of new analogs. Eur. J. Med. Chem. 2010, 45, 2972–2980. [Google Scholar] [CrossRef]
- Lappano, R.; Rosano, C.; Madeo, A.; Albanito, L.; Plastina, P.; Gabriele, B.; Forti, L.; Stivala, L.A.; Iacopetta, D.; Dolce, V.; et al. Structure-activity relationships of resveratrol and derivatives in breast cancer cells. Mol. Nutr. Food Res. 2009, 53, 845–858. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhu, Y.; Zhou, S.; An, X.; Zhang, Y.; Bai, Q.; He, Y.X.; Liu, H.; Yao, X. Experimental and theoretical insights into the inhibition mechanism of prion fibrillation by resveratrol and its derivatives. ACS Chem. Neurosci. 2017, 8, 2698–2707. [Google Scholar] [CrossRef] [PubMed]
- Albert, S.; Horbach, R.; Deising, H.B.; Siewert, B.; Csuk, R. Synthesis and antimicrobial activity of (E) stilbene derivatives. Bioorg. Med. Chem. 2011, 19, 5155–5166. [Google Scholar] [CrossRef] [PubMed]
- Csuk, R.; Albert, S.; Siewert, B.; Schwarz, S. Synthesis and biological evaluation of novel (E) stilbene-based antitumor agents. Eur. J. Med. Chem. 2012, 54, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Csuk, R.; Albert, S.; Kluge, R.; Ströhl, D. Resveratrol derived butyrylcholinesterase inhibitors. Arch. Pharm. Chem. Life Sci. 2013, 346, 499–503. [Google Scholar] [CrossRef]
- Csuk, R.; Albert, S.; Siewert, B. Synthesis and radical scavenging activities of resveratrol analogs. Arch. Pharm. Chem. Life Sci. 2013, 346, 504–510. [Google Scholar] [CrossRef]
- Fischer, N.; Büchter, C.; Koch, K.; Albert, S.; Csuk, R.; Wätjen, W. The resveratrol derivatives trans-3,5-dimethoxy-4-fluoro-4′-hydroxystilbene and trans-2,4′,5-trihydroxystilbene decrease oxidative stress and prolong lifespan in Caenorhabditis elegans. J. Pharm. Pharmacol. 2017, 69, 73–81. [Google Scholar] [CrossRef]
- Chalal, M.; Klinguer, A.; Echairi, A.; Meunier, P.; Vervandier-Fasseur, D.; Adrian, M. Antimicrobial activity of resveratrol analogues. Molecules 2014, 19, 7679–7688. [Google Scholar] [CrossRef]
- Chalal, M.; Delmas, D.; Meunier, P.; Latruffe, N.; Vervandier-Fasseur, D. Inhibition of cancer derived cell lines proliferation by newly synthesized hydroxylated stilbenes and ferrocenyl-stilbene analogs. Comparison with resveratrol. Molecules 2014, 19, 7850–7868. [Google Scholar] [CrossRef]
- Hauss, F.; Liu, J.; Michelucci, A.; Coowar, D.; Morga, E.; Heuschling, P.; Luu, B. Dual bioactivity of resveratrol fatty alcohols: Differentiation of neural stem cells and modulation of neuroinflammation. Bioorg. Med. Chem. Lett. 2007, 17, 4218–4222. [Google Scholar] [CrossRef]
- Villalonga-Barber, C.; Meligova, A.K.; Alexi, X.; Steele, B.R.; Kouzinos, C.E.; Screttas, C.G.; Katsanou, E.S.; Micha-Skrettas, M.; Alexis, M.N. New hydroxystilbenoid derivatives endowed with neuroprotective activity and devoid of interference with estrogen and aryl hydrocarbon receptor-mediated transcription. Bioorg. Med. Chem. 2011, 19, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, Y.; Sun, X.; Li, H.; LeSage, G.; Javer, A.; Zhang, X.; Wei, X.; Jiang, Y.; Yin, D. Synthetic resveratrol aliphatic acid inhibits TLR2-mediated apoptosis and an involvement of Akt/GSK3β pathway. Bioorg. Med. Chem. 2009, 17, 4378–4382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, L.F.; Wang, X.B.; Xie, S.S.; Li, S.Y.; Kong, L.Y. Multitarget-directed resveratrol derivatives: Anti-cholinesterases, anti-β-amyloid aggregation and monoamine oxidase inhibition properties against Alzheimer disease. MedChemComm 2014, 5, 609–616. [Google Scholar] [CrossRef]
- Liu, Q.; Kim, C.T.; Jo, Y.H.; Kim, S.B.; Hwang, B.Y.; Lee, M.K. Synthesis and biological evaluation of resveratrol derivatives as melanogenesis inhibitors. Molecules 2015, 20, 16933–16945. [Google Scholar] [CrossRef] [PubMed]
- Oh, W.Y.; Shahidi, F. Antioxidant activity of resveratrol ester derivatives in food and biological model systems. Food Chem. 2018, 261, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Biasutto, L.; Marotta, E.; De Marchi, U.; Zoratti, M.; Paradisi, C. Ester-based precursors to increase the bioavailability of quercetin. J. Med. Chem. 2007, 50, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Müller-Schiffmann, A.; Sticht, H.; Korth, C. Hybrid compounds: From simple combinations to nanomachines. BioDrugs 2012, 26, 21–31. [Google Scholar] [CrossRef]
- Li, W.; He, X.; Shi, W.; Jia, H.; Zhong, B. Pan-PPAR agonists based on the resveratrol scaffold: Biological evaluation and docking studies. ChemMedChem 2010, 5, 1977–1982. [Google Scholar] [CrossRef]
- Murty, M.S.R.; Penthala, R.; Polepalli, S.; Jain, N. Synthesis and biological evaluation of novel resveratrol-oxadiazole hybrid heterocycles as potential antiproliferative agents. Med. Chem. Res. 2016, 25, 627–643. [Google Scholar] [CrossRef]
- Peng, W.; Ma, Y.Y.; Zhang, K.; Zhou, A.Y.; Zhang, Y.; Wang, H.; Du, Z.; Zhao, D.G. Synthesis and biological evaluation of novel resveratrol-NSAID derivatives as anti-inflammatory agents. Chem. Pharm. Bull. 2016, 64, 609–615. [Google Scholar] [CrossRef] [Green Version]
- Bonechi, C.; Martini, S.; Ciani, L.; Lamponi, S.; Rebmann, H.; Rossi, C.; Ristori, S. Using liposomes as carriers for polyphenolic compounds: The case of trans-resveratrol. PLoS ONE 2012, 7, e41438. [Google Scholar] [CrossRef] [PubMed]
- Sciacca, M.F.M.; Chillemi, R.; Sciuto, S.; Pappalardo, M.; La Rosa, C.; Grasso, D.; Milardi, D. Interactions of two O-phosphorylresveratrol derivatives with model membranes. Arch. Biochem. Biophys. 2012, 521, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Chillemi, R.; Cardullo, N.; Greco, V.; Malfa, G.; Tomasello, B.; Sciuto, S. Synthesis of amphiphilic resveratrol lipoconjugates and evaluation of their anticancer activity towards neuroblastoma SH-SY5Y cell line. Eur. J. Med. Chem. 2015, 96, 467–481. [Google Scholar] [CrossRef] [PubMed]
- Sciacca, M.F.M.; Chillemi, R.; Sciuto, S.; Greco, V.; Messineo, C.; Kotler, S.A.; Lee, D.K.; Brender, J.R.; Ramamoorthy, A.; La Rosa, C.; et al. A blend of two resveratrol derivatives abolishes hIAPP amyloid growth and membrane damage. BBA Biomembranes 2018, 1860, 1793–1802. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.L. Design, synthesis and spectroscopic studies of resveratrol aliphatic acid ligands of human serum albumin. Bioorg. Med. Chem. 2008, 16, 6406–6414. [Google Scholar] [CrossRef] [PubMed]















© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Latruffe, N.; Vervandier-Fasseur, D. Strategic Syntheses of Vine and Wine Resveratrol Derivatives to Explore Their Effects on Cell Functions and Dysfunctions. Diseases 2018, 6, 110. https://doi.org/10.3390/diseases6040110
Latruffe N, Vervandier-Fasseur D. Strategic Syntheses of Vine and Wine Resveratrol Derivatives to Explore Their Effects on Cell Functions and Dysfunctions. Diseases. 2018; 6(4):110. https://doi.org/10.3390/diseases6040110
Chicago/Turabian StyleLatruffe, Norbert, and Dominique Vervandier-Fasseur. 2018. "Strategic Syntheses of Vine and Wine Resveratrol Derivatives to Explore Their Effects on Cell Functions and Dysfunctions" Diseases 6, no. 4: 110. https://doi.org/10.3390/diseases6040110
APA StyleLatruffe, N., & Vervandier-Fasseur, D. (2018). Strategic Syntheses of Vine and Wine Resveratrol Derivatives to Explore Their Effects on Cell Functions and Dysfunctions. Diseases, 6(4), 110. https://doi.org/10.3390/diseases6040110