Stability Enhancement and Skin Permeation Application of Nicotine by Forming Inclusion Complex with β-Cyclodextrin and Methyl-β-Cyclodextrin
Abstract
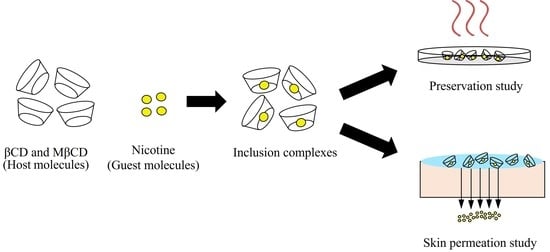
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Computational Simulation of Nicotine/CDs Inclusion Complex
2.3. Determination of the Host–Guest Molar Ratio of Nicotine/CDs Inclusion Complex by Job’s Method
2.4. Nicotine/CDs Inclusion Complex Preparation Method
2.5. Solvent Effect on the Encapsulation Efficiency of Nicotine/CDs Inclusion Complex
2.6. Nicotine/CDs Inclusion Complex Characterization
2.6.1. Gas Chromatography Coupled with Flame Ionization Detector (GC-FID)
2.6.2. Thermogravimetric Analysis (TGA)
2.6.3. Differential Scanning Calorimetry (DSC)
2.6.4. X-ray Diffractometry (XRD)
2.6.5. Fourier-Transform Infrared Spectroscopy (FTIR)
2.6.6. High-Performance Liquid Chromatography (HPLC)
2.7. Preservation Study of the Nicotine/CDs Inclusion Complex
2.8. Skin Permeation Study of the Nicotine/CDs Inclusion Complex
3. Results
3.1. Computational Simulation of the Nicotine/CDs Inclusion Complex
3.2. Determination of the Host–Guest Molar Ratio of the Nicotine/CDs Inclusion Complex by Job’s Method
3.3. Solvent Effect on the Encapsulation Efficiency of the Nicotine/CDs Inclusion Complex
3.4. Nicotine/CDs Inclusion Complex Characterization
3.4.1. Thermogravimetric Analysis (TGA)
3.4.2. Differential Scanning Calorimetry (DSC)
3.4.3. X-ray Diffractometry (XRD)
3.4.4. Fourier-Transform Infrared Spectroscopy (FTIR)
3.5. Preservation Study of Nicotine/CDs Inclusion Complex
3.6. Skin Permeation Study of the Nicotine/CDs Inclusion Complexes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Tayoub, G.; Sulaiman, H.; Alorfi, M. Determination of nicotine levels in the leaves of some Nicotiana tabacum varieties cultivated in Syria. Herba Pol. 2015, 61, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Benowitz, N.L. Pharmacology of nicotine: Addiction and therapeutics. Annu. Rev. Pharmacol. Toxicol. 1996, 36, 597–613. [Google Scholar] [CrossRef] [PubMed]
- Balandrin, M.F.; Klocke, J.A.; Wurtele, E.S.; Bollinger, W.H. Natural plant chemicals: Sources of industrial and medicinal materials. Science 1985, 228, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Powledge, T.M. Nicotine as therapy. PLoS Biol. 2004, 2, e404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newhouse, P.A.; Potter, A.; Kelton, M.; Corwin, J. Nicotinic treatment of Alzheimer’s disease. Biol. Psychiatry 2001, 49, 268–278. [Google Scholar] [CrossRef]
- Rusted, J.M.; Newhouse, P.A.; Levin, E.D. Nicotinic treatment for degenerative neuropsychiatric disorders such as Alzheimer’s disease and Parkinson’s disease. Behav. Brain Res. 2000, 113, 121–129. [Google Scholar] [CrossRef]
- White, H.K.; Levin, E.D. Four-week nicotine skin patch treatment effects on cognitive performance in Alzheimer’s disease. Psychopharmacology 1999, 143, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Hădărugă, D.I.; Hădărugă, N.G.; Butnaru, G.; Tatu, C.; Gruia, A. Bioactive microparticles (10): Thermal and oxidative stability of nicotine and its complex with β-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2010, 68, 155–164. [Google Scholar] [CrossRef]
- Del Valle, E.M. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Pinho, E.; Grootveld, M.; Soares, G.; Henriques, M. Cyclodextrins as encapsulation agents for plant bioactive compounds. Carbohydr. Polym. 2014, 101, 121–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ezhilarasi, P.; Karthik, P.; Chhanwal, N.; Anandharamakrishnan, C. Nanoencapsulation techniques for food bioactive components: A review. Food Bioproc. Technol. 2013, 6, 628–647. [Google Scholar] [CrossRef]
- Szente, L.; Szejtli, J. Highly soluble cyclodextrin derivatives: Chemistry, properties, and trends in development. Adv. Drug Deliv. Rev. 1999, 36, 17–28. [Google Scholar] [CrossRef]
- Berglund, J.; Cedergren, L.; Andersson, S.B. Determination of the stability constant for the inclusion complex between β-cyclodextrin and nicotine using capillary electrophoresis. Int. J. Pharm. 1997, 156, 195–200. [Google Scholar] [CrossRef]
- JS, P.; Kadam, D.; Marapur, S.; Kamalapur, M. Inclusion complex system; a novel technique to improve the solubility and bioavailability of poorly soluble drugs: A review. Int. J. Pharm. Sci. Rev. Res. 2010, 2, 29–34. [Google Scholar]
- Jiang, Z.; Tang, G.; Zhang, Y.; Zhao, J. catena-Poly [[dibromidomercury (II)]-μ-3-(1-methylpyrrolidin-2-yl) pyridine-κ2N: N′]. Acta Crystallogr. Sect. E Struct. Rep. Online 2008, 64, m1319. [Google Scholar] [CrossRef] [PubMed]
- Steiner, T.; Koellner, G. Crystalline. Beta.-cyclodextrin hydrate at various humidities: Fast, continuous, and reversible dehydration studied by X-ray diffraction. J. Am. Chem. Soc. 1994, 116, 5122–5128. [Google Scholar] [CrossRef]
- Manunza, B.; Deiana, S.; Pintore, M.; Gessa, C. Structure and internal motion of solvated beta-cyclodextrine: A molecular dynamics study. J. Mol. Struct. THEOCHEM 1997, 419, 133–137. [Google Scholar] [CrossRef]
- Biovia, D.S. Discovery Studio Visualizer; Dassault Systèmes: San Diego, CA, USA, 2017. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinlikhitkul, N.; Toochinda, P.; Lawtrakul, L.; Kuropakornpong, P.; Itharat, A. Encapsulation of plumbagin using cyclodextrins to enhance plumbagin stability: Computational simulation, preparation, characterization, and application. J. Incl. Phenom. Macrocycl. Chem. 2019, 93, 229–243. [Google Scholar] [CrossRef]
- Cilurzo, F.; Minghetti, P.; Sinico, C. Newborn pig skin as model membrane in in vitro drug permeation studies: A technical note. AAPS PharmSciTech 2007, 8, 97–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, A.; Jain, C.P.; Tanwar, Y.S. Preparation and characterization of solid dispersions of carvedilol with poloxamer 188. J. Chil. Chem. Soc. 2013, 58, 1553–1557. [Google Scholar] [CrossRef] [Green Version]
- Cui, F.; Li, Y.; Zhou, S.; Jia, M.; Yang, X.; Yu, F.; Ye, S.; Hou, Z.; Xie, L. A comparative in vitro evaluation of self-assembled PTX-PLA and PTX-MPEG-PLA nanoparticles. Nanoscale Res. Lett. 2013, 8, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| Orientation A | Orientation B | |
|---|---|---|
| Nicotine/βCD | −24.51 | −26.13 |
| Nicotine/MβCD | −17.41 | −22.73 |
| Inclusion Complex | Concentration of Ethanol (vol%) | Encapsulation Efficiency (%) |
|---|---|---|
| Nicotine/βCD | 0 | 59.96 ± 1.62 |
| 5 | 57.45 ± 1.06 | |
| 10 | 49.91 ± 1.26 | |
| Nicotine/MβCD | 0 | 63.76 ± 0.24 |
| 5 | 61.09 ± 1.25 | |
| 10 | 60.09 ± 1.26 |
| Sample Names | Amount of Nicotine (μg) | |
|---|---|---|
| After 30 min | After 60 min | |
| Nicotine/βCD gel | 4.08 ± 0.63 | 13.57 ± 0.18 |
| Nicotine/MβCD gel | 3.82 ± 0.67 | 9.81 ± 0.11 |
| Pure nicotine gel | 0.42 ± 0.02 | 0.95 ± 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chulurks, S.; Jitapunkul, K.; Katanyutanon, S.; Toochinda, P.; Lawtrakul, L. Stability Enhancement and Skin Permeation Application of Nicotine by Forming Inclusion Complex with β-Cyclodextrin and Methyl-β-Cyclodextrin. Sci. Pharm. 2021, 89, 43. https://doi.org/10.3390/scipharm89040043
Chulurks S, Jitapunkul K, Katanyutanon S, Toochinda P, Lawtrakul L. Stability Enhancement and Skin Permeation Application of Nicotine by Forming Inclusion Complex with β-Cyclodextrin and Methyl-β-Cyclodextrin. Scientia Pharmaceutica. 2021; 89(4):43. https://doi.org/10.3390/scipharm89040043
Chicago/Turabian StyleChulurks, Sorrawee, Kulpavee Jitapunkul, Sasimas Katanyutanon, Pisanu Toochinda, and Luckhana Lawtrakul. 2021. "Stability Enhancement and Skin Permeation Application of Nicotine by Forming Inclusion Complex with β-Cyclodextrin and Methyl-β-Cyclodextrin" Scientia Pharmaceutica 89, no. 4: 43. https://doi.org/10.3390/scipharm89040043
APA StyleChulurks, S., Jitapunkul, K., Katanyutanon, S., Toochinda, P., & Lawtrakul, L. (2021). Stability Enhancement and Skin Permeation Application of Nicotine by Forming Inclusion Complex with β-Cyclodextrin and Methyl-β-Cyclodextrin. Scientia Pharmaceutica, 89(4), 43. https://doi.org/10.3390/scipharm89040043