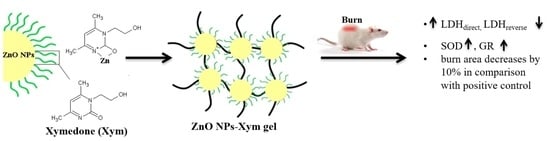
The Effect of Zinc Oxide Nanoparticles on Properties and Burn Wound Healing Activity of Thixotropic Xymedone Gels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of ZnO NPs
2.3. Synthesis of PEGylated ZnO NPs
2.4. Gels Preparations
2.5. FTIR Analysis
2.6. UV Analysis
2.7. Powder X-ray Diffraction Analysis
2.8. Zinc Assay
2.9. Photoluminescence Analysis
2.10. Viscosity Estimation
2.11. Surface Charge and Dynamic Light Scattering Measurements
2.12. SEM and EDXMA Studies
2.13. Specific Area Estimation
2.14. Permeability Study
2.15. Biological Activity
2.15.1. Modeling of Thermal Burns in Animals
2.15.2. Wound Area Measurement
2.15.3. Biological Activity
2.15.4. Assessment of Microcirculation in Burn Wound
2.16. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties of Modified ZnO NPs
3.2. Rheological Properties of Gels
3.3. Biomimetic Penetration of Xymedone and ZnO NPs–Xym from a 5% Gel through Theacetyl Cellulose Membrane
3.4. Biological Activity of the Pharmaceutical Composition ZnO NPs–Xym Gel Using the Thermal Burn Wound Model
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bano, T.; Kumar, N.; Dudhe, R. Free radical scavenging properties of pyrimidine derivatives. Org. Med. Chem. Lett. 2012, 14, 34. [Google Scholar] [CrossRef] [Green Version]
- He, Z.-X.; Zhao, T.-Q.; Gong, Y.-P.; Zhang, X.; Ma, L.-Y.; Liu, H.-M. Pyrimidine: A promising scaffold for optimization to develop the inhibitors of ABC transporters. Eur. J. Med. Chem. 2020, 200, 112458. [Google Scholar] [CrossRef]
- Izmailov, S.G.; Izmailov, G.A.; Averyanov, M.Y.; Reznik, V.S. Xymedon in Clinical Practice, 1st ed.; NNSMA: Nizhny Novgorod, Russia, 2001; p. 185. ISBN 5-7032-0112-8. [Google Scholar]
- Pradere, U.; Garnier-Amblard, E.C.; Coats, S.J.; Amblard, F.; Schinazi, R.F. Synthesis of nucleoside phosphate and phosphonate prodrugs. Chem. Rev. 2014, 114, 9154–9218. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Geng, H.; Junjie, Z.; He, K. An update mini-review on the progress of azanucleoside analogues. Chem. Pharm. Bull. 2022, 70, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Zvereva, E.E.; Vandyukova, I.I.; Vandyukov, A.E.; Katsyuba, S.A.; Khamatgalimov, A.R.; Kovalenko, V.I. IR and Raman spectra, hydrogen bonds, and conformations of N-(2-hydroxyethyl)-4,6-dimethyl-2-oxo-1,2-dihydropyrimidine (drug Xymedone). Russ. Chem. Bull. Int. Ed. 2012, 61, 1199–1206. [Google Scholar] [CrossRef]
- Beschastnov, V.V.; Izmailov, S.G.; Botyakov, A.A.; Zharinov, A.Y.; Panteleev, D.A.; Melnikova, N.B. Antioxidant activity of pyrimidine derivatives in the local treatment of purulent wounds of soft tissues (in experiment). Mod. Technol. Med. 2011, 3, 21–26. (In Russian) [Google Scholar]
- Krysanov, E.; Demidova, T.; Ivanova, O.; Ordzhonikidze, K.; Shcherbakov, A.; Ivanov, V. Synergetic action of ceria nanoparticles and doxorubicin on the early development of two fish species, Danio rerio and Puntius tetrazona. Nanosyst. Phys. Chem. Math. 2019, 10, 289–302. [Google Scholar] [CrossRef]
- Sack, M.; Alili, L.; Karaman, E.; Das, S.; Gupta, A.; Seal, S.; Brenneisen, P. Combination of Conventional Chemotherapeutics with Redox-Active Cerium Oxide Nanoparticles—A Novel Aspect in Cancer Therapy. Mol. Cancer Ther. 2014, 13, 1740–1749. [Google Scholar] [CrossRef] [Green Version]
- Popov, A.L.; Kolmanovich, D.D.; Popova, N.R.; Sorokina, S.S.; Ivanova, O.S.; Chukavin, N.N.; Shcherbakov, A.B.; Kozlova, T.O.; Kalashnikova, S.A.; Ivanov, V.K. Synthesis and biocompatibility study of ceria-mildronate nanocomposite in vitro. Nanosyst. Phys. Chem. Math. 2022, 13, 96–103. [Google Scholar] [CrossRef]
- Melnikova, N.; Orekhov, D.; Simagin, A.; Malygina, D.; Korokin, V.; Kosmachova, K.; Al-Azzavi, H.; Solovyeva, A.; Kazantsev, O. Antioxidant Activity of New Copolymer Conjugates of Methoxyoligo(Ethylene Glycol)Methacrylate and Betulin Methacrylate with Cerium Oxide Nanoparticles In Vitro. Molecules 2022, 27, 5894. [Google Scholar] [CrossRef]
- Gupta, M.; Mahajan, V.K.; Mehta, K.S.; Chauhan, P.S. Zinc Therapy in Dermatology: A Review. Dermatol. Res. Pract. 2014, 2014, 709152. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.M.; Song, Z.; Moghimi, H.R.; Roberts, M.S. Relative Penetration of Zinc Oxide and Zinc Ions into Human Skin after Application of Different Zinc Oxide Formulations. ACS Nano 2016, 10, 1810–1819. [Google Scholar] [CrossRef] [PubMed]
- Negut, I.; Grumezescu, V.; Grumezescu, A.M. Treatment Strategies for Infected Wounds. Molecules 2018, 23, 2392. [Google Scholar] [CrossRef] [Green Version]
- Melnikova, N.; Balakireva, A.; Orekhov, D.; Kamorin, D.; Didenko, N.; Malygina, D.; Knyazev, A.; Novopoltsev, D.; Solovyeva, A. Zinc Oxide Nanoparticles Protected with Terpenoids as a Substance in Redox Imbalance Normalization in Burns. Pharmaceuticals 2021, 14, 492. [Google Scholar] [CrossRef] [PubMed]
- Bera, D.; Qian, L.; Sabui, S.; Santra, A.; Holloway, P.H. Photoluminescence of ZnO quantum dots produced by a sol-gel process. Opt. Mater. 2008, 30, 1233–1239. [Google Scholar] [CrossRef]
- Pakhomova, A.E.; Pakhomova, E.E.; Pakhomova, J.V.; Yavorsky, E.M. Method Experimental Modeling of Thermal Combustion at Laboratory Animals. Patent No. RU 2,582,458 C1, 24 December 2014. [Google Scholar]
- Pakhomova, A.E.; Pakhomova, J.V.; Ovsyanko, E.V.; Zhurakovsky, I.P.; Karabintseva, N.O.; Pakhomova, E.E. Preclinical research of repalen ointment at treatment of thermal combustions in experiment. J. Sib. Med. Sci. 2015, 3, 98. [Google Scholar]
- Hosseinimehr, S.J.; Khorasani, G.; Azadbakht, M.; Zamani, P.; Ghasemi, M.; Ahmadi, A. Effect of aloe cream versus silver sulfadiazine for healing burn wounds in rats. Acta. Dermatovenerol. Croat. 2010, 18, 2–7. [Google Scholar]
- Mihara, M.; Uchiyama, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar] [PubMed]
- Sirota, T.V. A new approach to studying the autoxidation of adrenaline: Possibility of the determinationof superoxide dismutase activity and the antioxidant properties of various preparations by polarography. Biomed. Khi. 2012, 58, 77–87. [Google Scholar] [CrossRef] [Green Version]
- Packer, L. Catalase in Vitro. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Sibgatullina, G.V.; Khartendinova, L.R.; Gumerova, E.A.; Akulov, A.N.; Kostyukova, Y.A.; Nikonorova, N.A.; Rumyantseva, N.I. Methods for Determining the Redox Status of Cultured Plant Cells, 1st ed.; Kazan Federal University: Kazan, Russia, 2011; pp. 18–20. [Google Scholar]
- Solovyeva, A.G.; Zimin, Y.V. A new way to assess the dynamics of blood metabolism in patients with thermal trauma. Mod. Technol. Med. 2012, 2, 116–117. [Google Scholar]
- Dawson, J.M.; Heatlic, P.L. Lowry method of protein quantification evidence for photosensitivity. Anal. Biochem. 1984, 140, 391–393. [Google Scholar] [CrossRef]
- Guru, S.C.; Shetty, K.T. Methodological aspects of aldehyde dehydrogenase assay by spectrophotometric technique. Alcohol 1990, 7, 397–401. [Google Scholar] [CrossRef]
- Dahmus, J.D.; Bruning, R.S.; Kenney, W.L.; Alexander, L.M. Oral clopidogrel improves cutaneuos microvascular function through EDHF-dependent mechanisms in middle-aged humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R452–R458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krupatkin, A.I.; Sidorov, V.V. Functional Diagnostics of the State of Microcirculatory-Tissue Systems: Oscillations, Information, Nonlinearity: A Guide for Physicians, 1st ed.; Librokom: Moscow, Russia, 2013; p. 496. [Google Scholar]
- Stefanovska, A.; Bracic, M.; Desiree Kvernmo, H. Wavelet Analysis of Oscillations in the Peripheral Blood Circulation Measured by Laser Doppler Technique. IEEE Trans. Biomed. Eng. 1999, 46, 1230–1239. [Google Scholar] [CrossRef]








| Gel | Composition, wt.% | |||
|---|---|---|---|---|
| Carbopol 974 PNF | ZnO NPs | Xym | H2O | |
| ZnO NPs | 1.0 | 0.1 | - | up to 100 |
| ZnO NPs–Xym (5.0) | 1.0 | 0.1 | 5.0 | |
| ZnO NPs–Xym (2.5) | 0.5 | 0.05 | 2.5 | |
| Xym (5.0) | 1.0 | - | 5.0 | |
| Substance | ν, cm−1 | |||
|---|---|---|---|---|
| 3600–3200 (-OH, -NH and Other) | 3100–2900 (-CH, -CH2, -CH3) | 1655–1541 (-C=O, Amide I, Amide II) | 470–450 (Zn-O in ZnO NPs) | |
| ZnO NPs | 3468 | - | - | 451 |
| ZnO NPs–PEG | 3375 | - | - | 453 |
| ZnO NPs–Xym | 3274 | 3100–2500 | 1544 1606 1659 | 452 |
| Xymedone | 3209 | 3100–2500 | 1541 1602 1655 | - |
| Samples | Area under Curve | Hysteresis Loop | |
|---|---|---|---|
| Compression | Stretch | ||
| ZnO NPs (0.1%) gel | 124,648 | 130,479 | 5831 |
| ZnO NPs (0.05%)–Xym (2.5%) gel | 260,957 | 262,261 | 1304 |
| Xym (5%) gel | 117,904 | 116,890 | 1014 |
| Sample | Medium | Zeta Potential, mV |
|---|---|---|
| ZnO NPs | Ethanol:water (1:1) | +13.07 ± 1.20 |
| ZnO NPs | PBS | −1.30 ± 1.11 |
| ZnO NPs–Xym | PBS | −13.02 ± 2.79 |
| ZnO NPs–Xym * | PBS | −23.79 ± 3.55 |
| Group | Burn Area | |||
|---|---|---|---|---|
| 0 Day | 10 Day | |||
| cm2 | % of Control | cm2 | % of Control | |
| Burn untreated (negative control) | 23.97 ± 0.54 | 100 | 18.48 ± 0.35 | 77 |
| Methyluracyl®® (positive control) | 25.03 ± 0.48 | 100 | 15.01 ± 0.39 | 60 |
| ZnO NPs–Xym gel | 24.45 ± 0.25 | 100 | 12.75 ± 0.54 | 52 |
| ZnO NPs gel | 24.67 ± 0.23 | 100 | 16.94 ± 0.27 | 69 |
| Group | MI, perf. un | E, arb. un | N, arb. un | M, arb. un | R, arb. un | C, arb.un |
|---|---|---|---|---|---|---|
| Intact | 9.45 ± 0.85 | 12.93 ± 1.15 | 9.15 ± 0.86 | 8.37 ± 0.81 | 5.80 ± 0.49 | 3.33 ± 0.27 |
| Burn nontreatment (negative control) | 12.18 ± 1.11 | 14.04 ± 1.28 | 13.84 ± 1.26 | 15.34 ± 1.40 | 16.42 ± 1.49 | 9.50 ± 0.86 |
| Methyluracyl®® (positive control) | 10.36 ± 0.94 | 11.89 ± 1.08 | 11.94 ± 1.09 | 10.93 ± 0.99 | 10.87 ± 0.99 | 7.62 ± 0.69 |
| ZnO NPs–Xym gel | 9.40 ± 0.85 | 12.83 ± 1.17 | 10.73 ± 0.98 | 6.94 ± 0.63 | 7.34 ± 0.67 | 4.83 ± 0.44 |
| ZnO NPs gel | 10.01 ± 0.45 | 11.07 ± 0.98 | 12.13 ± 0.56 | 11.56 ± 0.98 | 12.49 ± 0.56 | 7.23 ± 0.72 |
| Group | Biochemical Indexes, % of Control | |||
|---|---|---|---|---|
| LDHdirect | LDHreverse | AlDH | MDAer | |
| Burn untreated (negative control) | 100.0 | 100.0 | 100.0 | 100.0 |
| Methyluracyl®® (positive control) | 122.47 | 142.58 | 125.75 | 105.89 |
| ZnO NPs–Xym gel | 142.02 | 99.32 | 124.04 | 102.21 |
| ZnO NPs gel | 111.52 | 135.29 | 110.93 | 115.35 |
| Group | Biochemical Indexes, % of Control | ||
|---|---|---|---|
| SOD | Catalase | GR | |
| Burn untreated (negative control) | 100.0 | 100.0 | 100.0 |
| Methyluracyl®® (positive control) | 130.78 | 105.16 | 183.44 |
| ZnO NPs–Xym gel | 135.02 | 104.50 | 160.26 |
| ZnO NPs gel | 110.28 | 98.39 | 120.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheferov, I.; Balakireva, A.; Panteleev, D.; Spitskaya, I.; Orekhov, S.; Kazantsev, O.; Solovyeva, A.; Novopoltsev, D.; Melnikova, N. The Effect of Zinc Oxide Nanoparticles on Properties and Burn Wound Healing Activity of Thixotropic Xymedone Gels. Sci. Pharm. 2022, 90, 61. https://doi.org/10.3390/scipharm90040061
Sheferov I, Balakireva A, Panteleev D, Spitskaya I, Orekhov S, Kazantsev O, Solovyeva A, Novopoltsev D, Melnikova N. The Effect of Zinc Oxide Nanoparticles on Properties and Burn Wound Healing Activity of Thixotropic Xymedone Gels. Scientia Pharmaceutica. 2022; 90(4):61. https://doi.org/10.3390/scipharm90040061
Chicago/Turabian StyleSheferov, Ilya, Alyona Balakireva, Dmitry Panteleev, Irina Spitskaya, Sergey Orekhov, Oleg Kazantsev, Anna Solovyeva, Denis Novopoltsev, and Nina Melnikova. 2022. "The Effect of Zinc Oxide Nanoparticles on Properties and Burn Wound Healing Activity of Thixotropic Xymedone Gels" Scientia Pharmaceutica 90, no. 4: 61. https://doi.org/10.3390/scipharm90040061
APA StyleSheferov, I., Balakireva, A., Panteleev, D., Spitskaya, I., Orekhov, S., Kazantsev, O., Solovyeva, A., Novopoltsev, D., & Melnikova, N. (2022). The Effect of Zinc Oxide Nanoparticles on Properties and Burn Wound Healing Activity of Thixotropic Xymedone Gels. Scientia Pharmaceutica, 90(4), 61. https://doi.org/10.3390/scipharm90040061