In Vitro Inhibitory Activities against α-Glucosidase, α-Amylase, and Pancreatic Lipase of Medicinal Plants Commonly Used in Chocó (Colombia) for Type 2 Diabetes and Obesity Treatment
Abstract
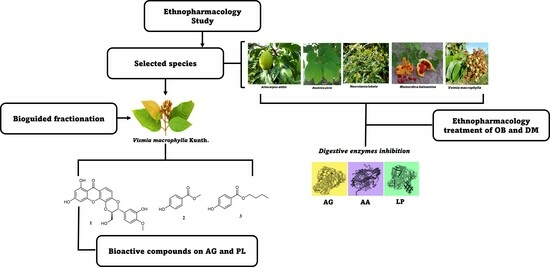
:1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Study Area
2.3. Ethnopharmacological Study and Determination of Use Value (UV) of Medicinal Plants
2.4. Plant Material
2.5. Hydroalcoholic Extracts (HE)
2.6. Digestive Enzyme Inhibition Assays
2.6.1. α-Glucosidase Inhibition Assay
2.6.2. α-Amylase Inhibition Assay
2.6.3. Pancreatic Lipase Inhibition Assay
2.7. Isolation of Bioactive Compounds from the Stem Bark of V. macrophylla
2.8. Enzyme Kinetic and Inhibition Mechanism Assay
2.9. Molecular Docking
2.10. Statistical Analysis
3. Results and Discussion
3.1. Ethnopharmacology of Diabetes and Obesity in Chocó, Colombia
3.2. Phytochemical Study of the Stem Bark of V. macrophylla and Enzymatic Inhibition against PL, AA, and AG
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, X.; Li, H. Obesity: Epidemiology, Pathophysiology, and Therapeutics. Front. Endocrinol. 2021, 12, 706978. [Google Scholar] [CrossRef] [PubMed]
- Avgerinos, K.I.; Spyrou, N.; Mantzoros, C.S.; Dalamaga, M. Obesity, and Cancer Risk: Emerging Biological Mecha-nisms and Perspectives. Metabolism 2019, 92, 121–135. [Google Scholar] [CrossRef]
- Piché, M.-E.; Tchernof, A.; Després, J.-P. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circ. Res. 2020, 126, 1477–1500. [Google Scholar] [CrossRef] [PubMed]
- Lobstein, T.; Brinsden, H.; Neveux, M. World Obesity Atlas. 2022. Available online: https://policycommons.net/artifacts/2266990/world_obesity_atlas_2022_web/3026660/?utm_medium=email&utm_source=transaction (accessed on 1 April 2023).
- Aras, M.; Tchang, B.G.; Pape, J. Obesity and Diabetes. Nurs. Clin. N. Am. 2021, 56, 527–541. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of Type 2 Diabetes—Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2020, 10, 107–111. [Google Scholar] [CrossRef]
- Atlas, I.D. IDF Atlas 10th Edition, International Diabetes Federation. IDF Diabetes Atlas. 2021. Available online: https://fmdiabetes.org/wp-content/uploads/2022/01/IDF_Atlas_10th_Edition_2021-comprimido.pdf (accessed on 1 April 2023).
- Sukhdev, S.; Bhupender, S.; Singh, K.S. Pharmacotherapy & Surgical Interventions Available for Obesity Manage-ment and Importance of Pancreatic Lipase Inhibitory Phytomolecules as Safer Anti-Obesity Therapeutics. Mini Rev. Med. Chem. 2017, 17, 371–379. [Google Scholar] [CrossRef]
- Inthongkaew, P.; Chatsumpun, N.; Supasuteekul, C.; Kitisripanya, T.; Putalun, W.; Likhitwitayawuid, K.; Sritu-larak, B. α-Glucosidase and Pancreatic Lipase Inhibitory Activities and Glucose Uptake Stimulatory Effect of Phenolic Compounds from Dendrobium Formosum. Rev. Bras. Farmacogn. 2017, 27, 480–487. [Google Scholar] [CrossRef]
- Hamden, K.; Mnafgui, K.; Amri, Z.; Aloulou, A.; Elfeki, A. Inhibition of Key Digestive Enzymes Related to Diabetes and Hyperlipidemia and Protection of Liver-Kidney Functions by Trigonelline in Diabetic Rats. Sci. Pharm. 2013, 81, 233–246. [Google Scholar] [CrossRef]
- Kato-Schwartz, C.G.; de Sá-Nakanishi, A.B.; Guidi, A.C.; Gonçalves, G.D.A.; Bueno, F.G.; Zani, B.P.M.; de Mello, J.C.P.; Bueno, P.S.A.; Seixas, F.A.V.; Bracht, A.; et al. Carbohydrate Digestive Enzymes Are Inhibited by Poincianella Plu-viosa Stem Bark Extract: Relevance on Type 2 Diabetes Treatment. Clin. Phytosci. 2020, 6, 31. [Google Scholar] [CrossRef]
- Lebovitz, H.E. alpha-Glucosidase inhibitors. Endocrinol. Metab. Clin. N. Am. 1997, 26, 539–551. [Google Scholar] [CrossRef]
- Gromova, L.V.; Polozov, A.S.; Savochkina, E.V.; Alekseeva, A.S.; Dmitrieva, Y.V.; Kornyushin, O.V.; Gruzdkov, A.A. Effect of Type 2 Diabetes and Impaired Glucose Tolerance on Digestive Enzymes and Glucose Absorption in the Small Intestine of Young Rats. Nutrients 2022, 14, 385. [Google Scholar] [CrossRef]
- Kaur, N.; Kumar, V.; Nayak, S.K.; Wadhwa, P.; Kaur, P.; Sahu, S.K. Alpha-amylase as molecular target for treatment of diabetes mellitus: A comprehensive review. Chem. Biol. Drug Des. 2021, 98, 539–560. [Google Scholar] [CrossRef]
- Pilitsi, E.; Farr, O.M.; Polyzos, S.A.; Perakakis, N.; Nolen-Doerr, E.; Papathanasiou, A.-E.; Mantzoros, C.S. Pharma-cotherapy of Obesity: Available Medications and Drugs under Investigation. Metabolism 2019, 92, 170–192. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.T.; Liu, X.T.; Chen, Q.X.; Shi, Y. Lipase Inhibitors for Obesity: A Review. Biomed. Pharmacother. 2020, 128, 110314. [Google Scholar] [CrossRef]
- Rajan, L.; Palaniswamy, D.; Mohankumar, S.K. Targeting obesity with plant-derived pancreatic lipase inhibitors: A comprehensive review. Pharmacol. Res. 2020, 155, 104681. [Google Scholar] [CrossRef]
- Hou, X.D.; Ge, G.B.; Weng, Z.M.; Dai, Z.R.; Leng, Y.H.; Ding, L.L.; Jin, L.L.; Yu, Y.; Cao, Y.F.; Hou, J. Natural constituents from Cortex Mori Radicis as new pancreatic lipase inhibitors. Bioorg. Chem. 2018, 80, 577–584. [Google Scholar] [CrossRef]
- Reed, J.; Bain, S.; Kanamarlapudi, V. A Review of Current Trends with Type 2 Diabetes Epidemiology, Aetiology, Pathogenesis, Treatments and Future Perspectives. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 3567–3602. [Google Scholar] [CrossRef]
- Quispe, Y.N.G.; Hwang, S.H.; Wang, Z.; Zuo, G.; Lim, S.S. Screening In Vitro Targets Related to Diabetes in Herbal Extracts from Peru: Identification of Active Compounds in Hypericum laricifolium Juss. by Offline High-Performance Liquid Chromatography. Int. J. Mol. Sci. 2017, 18, 2512. [Google Scholar] [CrossRef]
- Jugran, A.K.; Rawat, S.; Devkota, H.P.; Bhatt, I.D.; Rawal, R.S. Diabetes and Plant-Derived Natural Products: From Ethnopharmacological Approaches to Their Potential for Modern Drug Discovery and Development. Phytother. Res. PTR 2021, 35, 223–245. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E.A.; et al. Natural Products in Drug Discovery: Advances and Opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Shi, Y.; Burn, P. Lipid Metabolic Enzymes: Emerging Drug Targets for the Treatment of Obesity. Nat. Rev. Drug Discov. 2004, 3, 695–710. [Google Scholar] [CrossRef]
- Lunagariya, N.A.; Patel, N.K.; Jagtap, S.C.; Bhutani, K.K. Inhibitors of Pancreatic Lipase: State of the Art and Clini-cal Perspectives. EXCLI J. 2014, 13, 897–921. [Google Scholar] [PubMed]
- Huo, P.-C.; Hu, Q.; Shu, S.; Zhou, Q.-H.; He, R.-J.; Hou, J.; Guan, X.-Q.; Tu, D.-Z.; Hou, X.-D.; Liu, P.; et al. Design, Synthesis and Biological Evaluation of Novel Chalcone-like Compounds as Potent and Reversible Pancreatic Lipase Inhibitors. Bioorg. Med. Chem. 2021, 29, 115853. [Google Scholar] [CrossRef]
- Alhakamy, N.A.; Mohamed, G.A.; Fahmy, U.A.; Eid, B.G.; Ahmed, O.A.A.; Al-Rabia, M.W.; Khedr, A.I.M.; Nasrul-lah, M.Z.; Ibrahim, S.R.M. New Alpha-Amylase Inhibitory Metabolites from Pericarps of Garcinia Mangostana. Life 2022, 12, 384. [Google Scholar] [CrossRef]
- Tundis, R.; Loizzo, M.R.; Menichini, F. Natural Products as Alpha-Amylase and Alpha-Glucosidase Inhibitors and Their Hypoglycaemic Potential in the Treatment of Diabetes: An Update. Mini Rev. Med. Chem. 2010, 10, 315–331. [Google Scholar] [CrossRef]
- Chande, A.T.; Rowell, J.; Rishishwar, L.; Conley, A.B.; Norris, E.T.; Valderrama-Aguirre, A.; Medina-Rivas, M.A.; Jordan, I.K. Influence of Genetic Ancestry and Socioeconomic Status on Type 2 Diabetes in the Diverse Colombian Pop-ulations of Chocó and Antioquia. Sci. Rep. 2017, 7, 17127. [Google Scholar] [CrossRef]
- Cruz, E.C.; Andrade-Cetto, A. Ethnopharmacological Field Study of the Plants Used to Treat Type 2 Diabetes among the Cakchiquels in Guatemala. J. Ethnopharmacol. 2015, 159, 238–244. [Google Scholar] [CrossRef]
- Jenis, J.; Baiseitova, A.; Yoon, S.H.; Park, C.; Kim, J.Y.; Li, Z.P.; Lee, K.W.; Park, K.H. Competitive α-Glucosidase In-hibitors, Dihydrobenzoxanthones, from the Barks of Artocarpus Elasticus. J. Enzym. Inhib. Med. Chem. 2019, 34, 1623–1632. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Wang, R.; Han, L.; Huang, W.; Shi, H.; Wang, B.; Li, Z.; Zou, S. Altering the Inhibitory Kinetics and Molecular Conformation of Maltase by Tangzhiqing (TZQ), a Natural α-Glucosidase Inhibitor. BMC Complement. Med. Ther. 2020, 20, 350. [Google Scholar] [CrossRef]
- García-Cossio, F.; Terán, J.M.; Mena, X.B.; Palacios, V.M.; Mena, W.R.; Palacios, O.P. La Diabetes en Quibdó y su tratamiento. Rev. Inst. Univ. Tecnológica Chocó 2003, 18, 16–21. [Google Scholar]
- Cardozo-Muñoz, J.; Cuca-Suárez, L.E.; Prieto-Rodríguez, J.A.; Lopez-Vallejo, F.; Patiño-Ladino, O.J. Multitarget Action of Xanthones from Garcinia Mangostana against α-Amylase, α-Glucosidase and Pancreatic Lipase. Molecules 2022, 27, 3283. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.W.; Cho, J.K.; Curtis-Long, M.J.; Yuk, H.J.; Kim, Y.S.; Jung, S.; Kim, Y.S.; Lee, B.W.; Park, K.H. α-Glucosidase Inhibition and Antihyperglycemic Activity of Prenylated Xanthones from Garcinia Mangostana. Phytochemistry 2011, 72, 2148–2154. [Google Scholar] [CrossRef] [PubMed]
- Van Tilbeurgh, H.; Sarda, L.; Verger, R.; Cambillau, C. Structure of the Pancreatic Lipase-Procolipase Complex. Nature 1992, 359, 159–162. [Google Scholar] [CrossRef]
- Sim, L.; Quezada-Calvillo, R.; Sterchi, E.E.; Nichols, B.L.; Rose, D.R. Human Intestinal Maltase-Glucoamylase: Crystal Structure of the N-Terminal Catalytic Subunit and Basis of Inhibition and Substrate Specificity. J. Mol. Biol. 2008, 375, 782–792. [Google Scholar] [CrossRef]
- Zenderland, J.; Hart, R.; Bussmann, R.; Zambrana, N.; Sikharulidze, S.; Kikodze, D.; Tchelidze, D.; Khutsishvili, M.; Batsatsashvili, K. The Use of “Use Value”: Quantifying Importance in Ethnobotany. Econ. Bot. 2019, 73, 293–303. [Google Scholar] [CrossRef]
- Williams, L.K.; Li, C.; Withers, S.G.; Brayer, G.D. Order and Disorder: Differential Structural Impacts of Myricetin and Ethyl Caffeate on Human Amylase, an Antidiabetic Target. J. Med. Chem. 2012, 55, 10177–10186. [Google Scholar] [CrossRef]
- Friesner, R.A.; Banks, J.L.; Murphy, R.B.; Halgren, T.A.; Klicic, J.J.; Mainz, D.T.; Repasky, M.P.; Knoll, E.H.; Shelley, M.; Perry, J.K.; et al. Glide: A New Approach for Rapid, Accurate Docking and Scoring. 1. Method and Assessment of Docking Accuracy. J. Med. Chem. 2004, 47, 1739–1749. [Google Scholar] [CrossRef]
- Preto, J.; Gentile, F. Assessing and Improving the Performance of Consensus Docking Strategies Using the DockBox Package. J. Comput. Aided Mol. Des. 2019, 33, 817–829. [Google Scholar] [CrossRef]
- Hussain, H.; Hussain, J.; Al-Harrasi, A.; Saleem, M.; Green, I.R.; van Ree, T.; Ghulam, A. Chemistry and Biology of Genus Vismia. Pharm. Biol. 2012, 50, 1448–1462. [Google Scholar] [CrossRef]
- Andrade-Cetto, A.; Heinrich, M. From the Field into the Lab: Useful Approaches to Selecting Species Based on Local Knowledge. Front. Pharmacol. 2011, 2, 20. [Google Scholar] [CrossRef]
- Andrade-Cetto, A.; Cruz, E.C.; Cabello-Hernández, C.A.; Cárdenas-Vázquez, R. Hypoglycemic Activity of Medici-nal Plants Used among the Cakchiquels in Guatemala for the Treatment of Type 2 Diabetes. Evid.-Based Complement. Altern. Med. ECAM 2019, 2019, 2168603. [Google Scholar] [CrossRef]
- Dej-Adisai, S.; Pitakbut, T. Determination of A-Glucosidase Inhibitory Activity from Selected Fabaceae Plants. Pak. J. Pharm. Sci. 2015, 28, 1679–1683. [Google Scholar]
- Mukesh, S.; Sikarwar, B.H. Pharmacognostical, Phytochemical and Total Phenolic Content of Artocarpus Altilis (Parkinson) Fosberg Leaves. J. Appl. Pharm. Sci. 2015, 5, 94–100. [Google Scholar]
- Momina, S.; Rani, V. In Vitro Studies on α-Amylase and α-Glucosidase Inhibitory Activity of Some Bioactive Ex-tracts. J. Young Pharm. 2020, 12, s72–s75. [Google Scholar] [CrossRef]
- Haber, S.L.; Awwad, O.; Phillips, A.; Park, A.E.; Pham, T.M. Garcinia Cambogia for Weight Loss. Am. J. Health-Syst. Pharm. AJHP Off. J. Am. Soc. Health-Syst. Pharm. 2018, 75, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.-Y.; Mu, Q.; Gibbons, S. The Phytochemistry and Pharmacology of Hypericum. Prog. Chem. Org. Nat. Prod. 2020, 112, 85–182. [Google Scholar] [CrossRef] [PubMed]
- Vizcaya, M.; Morales, A.; Rojas, J.; Nunez, R. A Review on the Chemical Composition and Pharmacological Activi-ties of Vismia Genus (Guttiferae). Bol. Latinoam. Caribe Plantas Med. Aromat. 2012, 11, 12–34. [Google Scholar]
- Boonnak, N.; Khamthip, A.; Karalai, C.; Chantrapromma, S.; Ponglimanont, C.; Kanjana-Opas, A.; Tewtrakul, S.; Chantrapromma, K.; Fun, H.K.; Kato, S. Nitric Oxide Inhibitory Activity of Xanthones from the Green Fruits of Cratoxylum Formosum ssp. Pruniflorum. Aust. J. Chem. 2010, 63, 1550–1556. [Google Scholar] [CrossRef]
- Sim, W.C.; Ee, G.C.L.; Lim, C.J.; Sukari, M.A. Cratoxylum Glaucum and Cratoxylum Arborescens (Guttiferae)-Two Potential Source of Antioxidant Agents. Asian J. Chem. 2011, 23, 569–572. [Google Scholar]
- Sia, G.-L.; Bennett, G.J.; Harrison, L.J.; Sim, K.-Y. Minor Xanthones from the Bark of Cratoxylum Cochinchinense. Phytochemistry 1995, 38, 1521–1528. [Google Scholar] [CrossRef]
- Tian, J.-K.; Sun, F.; Cheng, Y.-Y. Chemical Constituents from the Roots of Ranunculus Ternatus. J. Asian Nat. Prod. Res. 2006, 8, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Inouye, M.; Chiba, J.; Nakazumi, H. Glucopyranoside Recognition by Polypyridine-Macrocyclic Receptors Pos-sessing a Wide Cavity with a Flexible Linkage. J. Org. Chem. 1999, 64, 8170–8176. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.-J.; Tsai, I.-L.; Chen, I.-S. Chemical Constituents from Strychnos Cathayensis. J. Chin. Chem. Soc. 2001, 48, 235–239. [Google Scholar] [CrossRef]
- Zhou, B.-D.; Weng, Z.-M.; Tong, Y.-G.; Ma, Z.-T.; Wei, R.-R.; Li, J.-L.; Yu, Z.-H.; Xu, G.-F.; Fang, Y.-Y.; Ruan, Z.-P. Syntheses of Xanthone Derivatives and Their Bioactivity Investigation. J. Asian Nat. Prod. Res. 2021, 23, 271–283. [Google Scholar] [CrossRef]
- Santos, C.M.M.; Freitas, M.; Fernandes, E. A Comprehensive Review on Xanthone Derivatives as α-Glucosidase Inhibitors. Eur. J. Med. Chem. 2018, 157, 1460–1479. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, L.; Chen, W.-H.; Park, H.; Ke, Z.; Wang, B. Binding Mechanism and Synergetic Effects of Xanthone De-rivatives as Noncompetitive α-Glucosidase Inhibitors: A Theoretical and Experimental Study. J. Phys. Chem. B 2013, 117, 13464–13471. [Google Scholar] [CrossRef]
- Cheng, Y.; Prusoff, W.H. Relationship between the Inhibition Constant (K1) and the Concentration of Inhibitor Which Causes 50 per Cent Inhibition (I50) of an Enzymatic Reaction. Biochem. Pharmacol. 1973, 22, 3099–3108. [Google Scholar] [CrossRef]
- Ramsay, R.R.; Tipton, K.F. Assessment of Enzyme Inhibition: A Review with Examples from the Development of Monoamine Oxidase and Cholinesterase Inhibitory Drugs. Molecules 2017, 22, 1192. [Google Scholar] [CrossRef]
- Geronimo, I.; Denning, C.A.; Heidary, D.K.; Glazer, E.C.; Payne, C.M. Molecular Determinants of Substrate Affinity and Enzyme Activity of a Cytochrome P450BM3 Variant. Biophys. J. 2018, 115, 1251–1263. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Schrödinger Release 2022-1: Maestro, Schrödinger, LLC, New York, NY. 2021. Available online: https://www.schrodinger.com/ (accessed on 10 December 2021).
- Sankar, V.; Maida Engels, S.E. Synthesis, Biological Evaluation, Molecular Docking and in Silico ADME Studies of Phenacyl Esters of N-Phthaloyl Amino Acids as Pancreatic Lipase Inhibitors. Future J. Pharm. Sci. 2018, 4, 276–283. [Google Scholar] [CrossRef]
- Chahinian, H.; Bezzine, S.; Ferrato, F.; Ivanova, M.G.; Perez, B.; Lowe, M.E.; Carrière, F. The Β5’ Loop of the Pancre-atic Lipase C2-like Domain Plays a Critical Role in the Lipase−Lipid Interactions. Biochemistry 2002, 41, 13725–13735. [Google Scholar] [CrossRef] [PubMed]
- Almasri, I.M. Computational Approaches for the Discovery of Natural Pancreatic Lipase Inhibitors as Antiobesity Agents. Future Med. Chem. 2020, 12, 741–757. [Google Scholar] [CrossRef] [PubMed]




| Species | Family | Common Name | Used Part | FTP | TU | UV |
|---|---|---|---|---|---|---|
| Justicia chlorostachya | Acanthaceae | Insulina | Leaves | D and I | DM | 0.48 |
| Trichanthera gigantea | Acanthaceae | Quiebra barriga | Leaves | D and I | OB | 0.52 |
| Amaranthus sp. | Amarantaceae | Amaranto | Leaves | D | OB | 0.56 |
| Iresine herbstii | Amarantaceae | Escancel | Leaves | D and I | DM | 0.52 |
| Anacardium occidentale | Anacardiaceae | Mango | Leaves | D and I | OB and DM | 0.48 |
| Annona muricata | Annonaceae | Guanábana | Fruits and leaves | D and J | DM | 0.48 |
| Neurolaena lobata | Asteraceae | Venadillo | Whole plant | D | DM | 0.76 |
| Bidens pilosa | Asteraceae | Pacunga | Whole plant | D and I | DM | 0.48 |
| Spilanthes paniculata | Asteraceae | Botoncillo | Leaves | D | OB | 0.52 |
| Bauhinia picta | Fabaceae | Casco de vaca | Leaves | D and I | DM | 0.64 |
| Vismia macrophylla | Hypericaceae | Manchará | Leaves and bark | D and I | OB and DM | 0.64 |
| Momordica balsamina | Cucurbitaceae | Balsamina | Leaves | D | OB and DM | 0.72 |
| Kyllinga pumila | Cyperaceae | Espadilla | Leaves | D and I | OB and DM | 0.56 |
| Phaseolus vulgaris | Fabaceae | Frijol | Fruits and leaves | D and I | DM | 0.48 |
| Origanum vulgare | Lamiaceae | Orégano | Whole plant | D and I | DM | 0.48 |
| Plectranthus amboinicus | Lamiaceae | Orégano brujo | Whole plant | D and I | DM | 0.48 |
| Sida rhombifolia | Malvaceae | Escoba babosa | Leaves | D and I | OB and DM | 0.48 |
| Bellucia pentamera | Melastomataceae | Coronillo | Fruits and leaves | D and J | OB | 0.52 |
| Artocarpus altilis | Moraceae | Árbol del pan | Leaves | D | DM | 0.80 |
| Syzygium malaccense | Myrtaceae | Marañón | Fruits and leaves | D and J | OB and D | 0.56 |
| Eucalyptus globulus | Myrtaceae | Eucalipto | Leaves and bark | D and I | DM | 0.48 |
| Psidium guajava | Myrtaceae | Guayaba | Fruits and leaves | D and J | OB and DM | 0.52 |
| Averrhoa carambola | Oxalydaceae | Carambolo | Fruits and leaves | D and J | OB | 0.48 |
| Passiflora quadrangularis | Passifloraceae | Badea | Leaves | D and I | OB and DM | 0.56 |
| Peperomia pellucida | Piperaceae | Celedonia | Whole plant | D and I | OB | 0.60 |
| Scoparia dulcis | Plantaginaceae | Escubilla | Leaves | D and I | OB | 0.48 |
| Cymbopogon citratus | Poaceae | Limoncillo | Whole plant | D | DM | 0.52 |
| Physalis peruviana | Solanaceae | Uchuva | Fruits and leaves | D and I | DM | 0.60 |
| Pilea microphylla | Urticaceae | Cien piecitos | Whole plant | D | OB and DM | 0.48 |
| Extracts | AG IC50 ± SD (µg/mL) | AA IC50 ± SD (mg/mL) | PL IC50 ± SD (µg/mL) |
|---|---|---|---|
| B. picta | 35.80 ± 3.2 | 7.34 ± 0.36 | 64.28 ± 5.20 |
| M. balsamina | 123.30 ± 2.5 | NA | 22.85 ± 7.28 * |
| N. lobata | 71.23± 1.8 | 37.80 ± 2.43 | 17.18 ± 2.55 * |
| A. altilis | 55.07 ± 2.9 | 24.48 ± 1.50 | 30.46 ± 5.24 |
| V. macrophylla stem bark | 0.99 ± 0.12 ** | 5.61 ± 0.82 | 28.91 ± 2.10 |
| V. macrophylla leaves | 5.74 ± 1.55 * | 4.23 ± 0.24 | 250.00 ± 5.40 |
| a Acarbose | 104.00 ± 2.9 | 0.80 ± 0.04 ** | |
| b Orlistat | 4.41 ± 2.04 * |
| Extracts/Fractions | AG IC50 ± SD (µg/mL) | AA IC50 ± SD (mg/mL) | PL IC50 ± SD (µg/mL) |
|---|---|---|---|
| V. macrophylla Stem bark HE | 0.99 ± 0.21 *** | 5.61 ± 0.82 | 28.91 ± 2.10 * |
| n-hexane fraction | NA | NA | 9.37 ± 3.00 ** |
| CHCl3 fraction | 3.47 ± 1.08 *** | 6.80 ± 0.92 | 26.53 ± 5.38 * |
| EtOAc fraction | 0.59 ± 0.10 *** | 1.96 ± 0.62 ** | 68.84 ± 1.00 |
| a Acarbose | 104.00 ± 2.9 | 0.80 ± 0.04 *** | |
| b Orlistat | 4.41 ± 2.04 ** |
| Compounds | α-Glucosidase | Pancreatic Lipase | ||||
|---|---|---|---|---|---|---|
| IC50 (µM) | ki μM | Inhibitor Type | IC50 (µM) | ki μM | Inhibitor Type | |
| 1 | 164.30 ± 0.11 * | 131.70 | C | 187.50 ± 0.33 | 187.50 | M |
| 2 | >400 | - | - | 28.50 ± 4.07 * | 10.60 | NC |
| 3 | >400 | - | - | 10.15 ± 3.42 ** | 5.31 | NC |
| Acarbose | 315.20 ± 3.27 | 57.50 | C | - | - | |
| Orlistat | - | 0.67 ± 0.04 *** | 0.24 | II | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lévuok-Mena, K.P.; Patiño-Ladino, O.J.; Prieto-Rodríguez, J.A. In Vitro Inhibitory Activities against α-Glucosidase, α-Amylase, and Pancreatic Lipase of Medicinal Plants Commonly Used in Chocó (Colombia) for Type 2 Diabetes and Obesity Treatment. Sci. Pharm. 2023, 91, 49. https://doi.org/10.3390/scipharm91040049
Lévuok-Mena KP, Patiño-Ladino OJ, Prieto-Rodríguez JA. In Vitro Inhibitory Activities against α-Glucosidase, α-Amylase, and Pancreatic Lipase of Medicinal Plants Commonly Used in Chocó (Colombia) for Type 2 Diabetes and Obesity Treatment. Scientia Pharmaceutica. 2023; 91(4):49. https://doi.org/10.3390/scipharm91040049
Chicago/Turabian StyleLévuok-Mena, Kevin P., Oscar J. Patiño-Ladino, and Juliet A. Prieto-Rodríguez. 2023. "In Vitro Inhibitory Activities against α-Glucosidase, α-Amylase, and Pancreatic Lipase of Medicinal Plants Commonly Used in Chocó (Colombia) for Type 2 Diabetes and Obesity Treatment" Scientia Pharmaceutica 91, no. 4: 49. https://doi.org/10.3390/scipharm91040049
APA StyleLévuok-Mena, K. P., Patiño-Ladino, O. J., & Prieto-Rodríguez, J. A. (2023). In Vitro Inhibitory Activities against α-Glucosidase, α-Amylase, and Pancreatic Lipase of Medicinal Plants Commonly Used in Chocó (Colombia) for Type 2 Diabetes and Obesity Treatment. Scientia Pharmaceutica, 91(4), 49. https://doi.org/10.3390/scipharm91040049