Antifungal Drugs
Abstract
:1. Introduction
2. Overview of Antifungal Agents and Their Mechanisms of Action
3. Pharmacology and Toxicity of Antifungal Agents
4. Susceptibility of Antifungal Agents
5. Resistance to Antifungal Agents
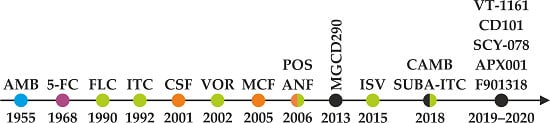
6. The Antifungal Pipeline
Author Contributions
Funding
Conflicts of Interest
References
- Pianalto, K.; Alspaugh, J.A. New Horizons in Antifungal Therapy. J. Fungi 2016, 2, 26. [Google Scholar] [CrossRef]
- Hidden Crisis: How 150 People Die Every Hour from Fungal Infection While the World Turns a Blind Eye. Available online: https://www.gaffi.org/wp-content/uploads/GAFFI-Leaflet-June-2016-DWD-hidden-crisis.pdf (accessed on 3 March 2020).
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases—Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef]
- Prakash, H.; Chakrabarti, A. Global Epidemiology of Mucormycosis. J. Fungi 2019, 5, 26. [Google Scholar] [CrossRef] [Green Version]
- Brown, G.D.; Denning, D.W.; Gow, N.; Levitz, S.M.; Netea, M.; White, T.C. Hidden Killers: Human Fungal Infections. Sci. Transl. Med. 2012, 4, 165rv13. [Google Scholar] [CrossRef] [Green Version]
- Guarro, J.; Kantarcioglu, A.S.; Horré, R.; Rodríguez-Tudela, J.L.; Estrella, M.C.; Berenguer, J.; De Hoog, G.S. Scedosporium apiospermum: Changing clinical spectrum of a therapy-refractory opportunist. Med. Mycol. 2006, 44, 295–327. [Google Scholar] [CrossRef] [Green Version]
- Nucci, M.; Anaissie, E. Fusarium Infections in Immunocompromised Patients. Clin. Microbiol. Rev. 2007, 20, 695–704. [Google Scholar] [CrossRef] [Green Version]
- Carmona, E.M.; Limper, A.H. Overview of Treatment Approaches for Fungal Infections. Clin. Chest Med. 2017, 38, 393–402. [Google Scholar] [CrossRef]
- Kumar, A.; Zarychanski, R.; Pisipati, A.; Kumar, A.; Kethireddy, S.; Bow, E.J. Fungicidal versus fungistatic therapy of invasiveCandidainfection in non-neutropenic adults: A meta-analysis. Mycology 2018, 9, 116–128. [Google Scholar] [CrossRef] [Green Version]
- Meletiadis, J.; Antachopoulos, C.; Stergiopoulou, T.; Pournaras, S.; Roilides, E.; Walsh, T.J. Differential Fungicidal Activities of Amphotericin B and Voriconazole against Aspergillus Species Determined by Microbroth Methodology. Antimicrob. Agents Chemother. 2007, 51, 3329–3337. [Google Scholar] [CrossRef] [Green Version]
- Geißel, B.; Loiko, V.; Klugherz, I.; Zhu, Z.; Wagener, N.; Kurzai, O.; Hondel, C.A.M.J.J.V.D.; Wagener, J. Azole-induced cell wall carbohydrate patches kill Aspergillus fumigatus. Nat. Commun. 2018, 9, 3098. [Google Scholar] [CrossRef] [Green Version]
- Patil, A.; Majumdar, S. Echinocandins in antifungal pharmacotherapy. J. Pharm. Pharmacol. 2017, 69, 1635–1660. [Google Scholar] [CrossRef] [Green Version]
- Revie, N.M.; Iyer, K.R.; Robbins, N.; Cowen, L. Antifungal drug resistance: Evolution, mechanisms and impact. Curr. Opin. Microbiol. 2018, 45, 70–76. [Google Scholar] [CrossRef]
- Ten Threats to Global Health in 2019. Available online: https://www.who.int/emergencies/ten-threats-to-global-health-in-2019 (accessed on 3 June 2019).
- Ahmad, S.; Bhattacharya, D.; Kar, S.; Ranganathan, A.; Van Kaer, L.; Das, G. Curcumin Nanoparticles Enhance Mycobacterium bovis BCG Vaccine Efficacy by Modulating Host Immune Responses. Infect. Immun. 2019, 87, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Scriven, J.E.; Tenforde, M.W.; Levitz, S.M.; Jarvis, J.N. Modulating host immune responses to fight invasive fungal infections. Curr. Opin. Microbiol. 2017, 40, 95–103. [Google Scholar] [CrossRef] [Green Version]
- van de Sande, W.; Vonk, A.G. Mycovirus therapy for invasive pulmonary aspergillosis? Med. Mycol. 2019, 57, S179–S188. [Google Scholar] [CrossRef]
- Nerva, L.; Chitarra, W.; Siciliano, I.; Gaiotti, F.; Ciuffo, M.; Forgia, M.; Varese, G.C.; Turina, M. Mycoviruses mediate mycotoxin regulation in Aspergillus ochraceus. Environ. Microbiol. 2018, 21, 1957–1968. [Google Scholar] [CrossRef] [Green Version]
- Zotchev, S.B. Polyene macrolide antibiotics and their applications in human therapy. Curr. Med. Chem. 2003, 10, 211–223. [Google Scholar] [CrossRef]
- Vermes, A.; Guchelaar, H.-J.; Dankert, J. Flucytosine: A review of its pharmacology, clinical indications, pharmacokinetics, toxicity and drug interactions. J. Antimicrob. Chemother. 2000, 46, 171–179. [Google Scholar] [CrossRef]
- Ping, B.; Zhu, Y.; Gao, Y.; Yue, C.; Wu, B. Second- versus first-generation azoles for antifungal prophylaxis in hematology patients: A systematic review and meta-analysis. Ann. Hematol. 2013, 92, 831–839. [Google Scholar] [CrossRef]
- Livengood, S.J.; Drew, R.H.; Perfect, J.R. Combination Therapy for Invasive Fungal Infections. Curr. Fungal Infect. Rep. 2020, 14, 40–49. [Google Scholar] [CrossRef]
- Mesa-Arango, A.C.; Scorzoni, L.; Zaragoza, O. It only takes one to do many jobs: Amphotericin B as antifungal and immunomodulatory drug. Front. Microbiol. 2012, 3, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Tevyashova, A.N.; Olsufyeva, E.; Solovieva, S.E.; Printsevskaya, S.S.; Reznikova, M.I.; Trenin, A.S.; Galatenko, O.A.; Treshalin, I.D.; Pereverzeva, E.R.; Mirchink, E.P.; et al. Structure-Antifungal Activity Relationships of Polyene Antibiotics of the Amphotericin B Group. Antimicrob. Agents Chemother. 2013, 57, 3815–3822. [Google Scholar] [CrossRef] [Green Version]
- Parker, W.B. Enzymology of Purine and Pyrimidine Antimetabolites Used in the Treatment of Cancer. Chem. Rev. 2009, 109, 2880–2893. [Google Scholar] [CrossRef] [Green Version]
- Sagatova, A.A.; Keniya, M.V.; Wilson, R.K.; Monk, B.C.; Tyndall, J. Structural Insights into Binding of the Antifungal Drug Fluconazole to Saccharomyces cerevisiae Lanosterol 14α-Demethylase. Antimicrob. Agents Chemother. 2015, 59, 4982–4989. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Balasubramanian, M.K. 1,3-beta-Glucan synthase: A useful target for antifungal drugs. Curr. Drug Target Infect. Disord. 2001, 1, 159–169. [Google Scholar] [CrossRef]
- Yao, J.; Liu, H.; Zhou, T.; Chen, H.; Miao, Z.; Sheng, C.; Zhang, W. Total synthesis and structure–activity relationships of new echinocandin-like antifungal cyclolipohexapeptides. Eur. J. Med. Chem. 2012, 50, 196–208. [Google Scholar] [CrossRef]
- Hamill, R.J. Amphotericin B Formulations: A Comparative Review of Efficacy and Toxicity. Drugs 2013, 73, 919–934. [Google Scholar] [CrossRef]
- Momparler, R.L. Optimization of cytarabine (ARA-C) therapy for acute myeloid leukemia. Exp. Hematol. Oncol. 2013, 2, 20. [Google Scholar] [CrossRef] [Green Version]
- Nett, J.E.; Andes, D.R. Antifungal Agents. Infect. Dis. Clin. North Am. 2016, 30, 51–83. [Google Scholar] [CrossRef]
- Debruyne, D.; Ryckelynck, J.-P. Clinical Pharmacokinetics of Fluconazole. Clin. Pharmacokinet. 1993, 24, 10–27. [Google Scholar] [CrossRef]
- Heykants, J.; Van Peer, A.; Van De Velde, V.; Van Rooy, P.; Meuldermans, W.; Lavrijsen, K.; Woestenborghs, R.; Van Cutsem, J.; Cauwenbergh, G. The Clinical Pharmacokinetics of Itraconazole: An Overview. Mycoses 1989, 32, 67–87. [Google Scholar] [CrossRef]
- Salavert, M.; Jarque, I.; Zaragoza, R.; Gobernado, M. Voriconazole in the management of nosocomial invasive fungal infections. Ther. Clin. Risk Manag. 2006, 2, 129–157. [Google Scholar]
- Li, Y.; Theuretzbacher, U.; Clancy, C.J.; Nguyen, M.H.; Derendorf, H.; Derendorf, H. Pharmacokinetic/Pharmacodynamic Profile of Posaconazole. Clin. Pharmacokinet. 2010, 49, 379–396. [Google Scholar] [CrossRef]
- Rybak, J.M.; Marx, K.R.; Nishimoto, A.T.; Rogers, P.D. Isavuconazole: Pharmacology, Pharmacodynamics, and Current Clinical Experience with a New Triazole Antifungal Agent. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2015, 35, 1037–1051. [Google Scholar] [CrossRef]
- Kofla, G.; Ruhnke, M. Pharmacology and metabolism of anidulafungin, caspofungin and micafungin in the treatment of invasive candidosis—review of the literature. Eur. J. Med Res. 2011, 16, 159–166. [Google Scholar] [CrossRef] [Green Version]
- Bellmann, R.; Smuszkiewicz, P. Pharmacokinetics of antifungal drugs: Practical implications for optimized treatment of patients. Infection 2017, 45, 737–779. [Google Scholar] [CrossRef]
- ERAXIS Product Monograph. Anidulafungin for Injection 100 mg/vial. Available online: https://www.pfizer.ca/sites/default/files/201710/ERAXIS_PM_E_176889_14Oct2014.pdf (accessed on 28 February 2020).
- CANDIDAS Product Monograph. Caspofungin for Injection 50 mg/vial, 70 mg/vial. Available online: https://www.merck.ca/static/pdf/CANCIDAS-PM_E.pdf (accessed on 28 February 2020).
- Mycamine Product Monograph. Micafungin Sodium for Injection 50 mg and 100 mg/vial. Available online: https://pdf.hres.ca/dpd_pm/00024563.PDF (accessed on 28 February 2020).
- Bersani, I.; Piersigilli, F.; Goffredo, B.M.; Santisi, A.; Cairoli, S.; Ronchetti, M.P.; Auriti, C. Antifungal Drugs for Invasive Candida Infections (ICI) in Neonates: Future Perspectives. Front. Pediatr. 2019, 7, 375. [Google Scholar] [CrossRef] [Green Version]
- Warris, A.; Lehrnbecher, T.; Roilides, E.; Castagnola, E.; Bruggemann, R.J.; Groll, A.H. ESCMID-ECMM guideline: Diagnosis and management of invasive aspergillosis in neonates and children. Clin. Microbiol. Infect. 2019, 25, 1096–1113. [Google Scholar] [CrossRef] [Green Version]
- Cornely, O.A.; Bassetti, M.; Calandra, T.; Garbino, J.; Kullberg, B.; Lortholary, O.; Meersseman, W.; Akova, M.; Arendrup, M.C.; Arikan-Akdagli, S.; et al. ESCMID This guideline was presented in part at ECCMID 2011. European Society for Clinical Microbiology and Infectious Diseases. guideline for the diagnosis and management of Candida diseases 2012: Non-neutropenic adult patients. Clin. Microbiol. Infect. 2012, 18, 19–37. [Google Scholar] [CrossRef] [Green Version]
- Ullmann, A.; Aguado, J.; Arikan-Akdagli, S.; Denning, D.; Groll, A.; Lagrou, K.; Lass-Flörl, C.; Lewis, R.; Munoz, P.; Verweij, P.E.; et al. Diagnosis and management of Aspergillus diseases: Executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin. Microbiol. Infect. 2018, 24, e1–e38. [Google Scholar] [CrossRef]
- Pilmis, B.; Jullien, V.; Sobel, J.; Lecuit, M.; Lortholary, O.; Charlier, C. Antifungal drugs during pregnancy: An updated review. J. Antimicrob. Chemother. 2014, 70, 14–22. [Google Scholar] [CrossRef] [Green Version]
- Payne, K.D.; Hall, R. Dosing of Antifungal Agents in Obese People. Expert Rev. Anti Infect. Ther. 2015, 14, 257–267. [Google Scholar] [CrossRef]
- Lestner, J.; Smith, P.B.; Cohen-Wolkowiez, M.; Benjamin, D.K.; Hope, W. Antifungal agents and therapy for infants and children with invasive fungal infections: A pharmacological perspective. Br. J. Clin. Pharmacol. 2012, 75, 1381–1395. [Google Scholar] [CrossRef]
- EUCAST. Available online: http://www.eucast.org/ (accessed on 24 January 2020).
- CLSI. Available online: https://clsi.org/ (accessed on 24 January 2020).
- Alastruey-Izquierdo, A.; Melhem, M.; Bonfietti, L.X.; Rodriguez-Tudela, J.L. Susceptibility test for fungi: Clinical and laboratorial correlations in medical mycology. Rev. Inst. Med. Trop. São Paulo 2015, 57, 57–64. [Google Scholar] [CrossRef] [Green Version]
- New Definitions of S, I and R from 2019. Available online: http://www.eucast.org/newsiandr/ (accessed on 23 January 2020).
- Kahlmeter, G.; Giske, C.G.; Kirn, T.J.; Sharp, S.E. Point-Counterpoint: Differences between the European Committee on Antimicrobial Susceptibility Testing and Clinical and Laboratory Standards Institute Recommendations for Reporting Antimicrobial Susceptibility Results. J. Clin. Microbiol. 2019, 57, e01129-19. [Google Scholar] [CrossRef] [Green Version]
- Sanguinetti, M.; Posteraro, B. Susceptibility Testing of Fungi to Antifungal Drugs. J. Fungi 2018, 4, 110. [Google Scholar] [CrossRef] [Green Version]
- Breakpoint Tables for Interpretation of MICs for Antifungal Agents, Version 10.0. Available online: http://www.eucast.org/astoffungi/clinicalbreakpointsforantifungals/ (accessed on 28 February 2020).
- Tortorano, A.M.; Rautemaa-Richardson, R.; Roilides, E.; Van Diepeningen, A.; Caira, M.; Muñoz, P.; Johnson, E.; Meletiadis, J.; Pana, Z.-D.; Lackner, M.; et al. ESCMID and ECMM joint guidelines on diagnosis and management of hyalohyphomycosis: Fusarium spp., Scedosporium spp. and others. Clin. Microbiol. Infect. 2014, 20, 27–46. [Google Scholar] [CrossRef] [Green Version]
- Sanguinetti, M.; Posteraro, B.; Lass-Flörl, C. Antifungal drug resistance amongCandidaspecies: Mechanisms and clinical impact. Mycoses 2015, 58, 2–13. [Google Scholar] [CrossRef]
- Morio, F.; Jensen, R.H.; Le Pape, P.; Arendrup, M.C. Molecular basis of antifungal drug resistance in yeasts. Int. J. Antimicrob. Agents 2017, 50, 599–606. [Google Scholar] [CrossRef]
- Beardsley, J.; Halliday, C.L.; Chen, S.C.-A.; Sorrell, T. Responding to the emergence of antifungal drug resistance: Perspectives from the bench and the bedside. Futur. Microbiol. 2018, 13, 1175–1191. [Google Scholar] [CrossRef] [Green Version]
- Verweij, P.E.; Chowdhary, A.; Melchers, W.J.G.; Meis, J.F. Azole Resistance in Aspergillus fumigatus: Can We Retain the Clinical Use of Mold-Active Antifungal Azoles? Clin. Infect. Dis. 2015, 62, 362–368. [Google Scholar] [CrossRef] [Green Version]
- Berger, S.; El Chazli, Y.; Babu, A.F.; Coste, A. Azole Resistance in Aspergillus fumigatus: A Consequence of Antifungal Use in Agriculture? Front. Microbiol. 2017, 8, 1024–1030. [Google Scholar] [CrossRef] [Green Version]
- Cutler, J.E.; Corti, M.; Lambert, P.; Ferris, M.; Xin, H. Horizontal Transmission of Candida albicans and Evidence of a Vaccine Response in Mice Colonized with the Fungus. PLoS ONE 2011, 6, 22030. [Google Scholar] [CrossRef]
- Bliss, J.M.; Basavegowda, K.P.; Watson, W.J.; Sheikh, A.U.; Ryan, R.M. Vertical and Horizontal Transmission of Candida albicans in Very Low Birth Weight Infants Using DNA Fingerprinting Techniques. Pediatr. Infect. Dis. J. 2008, 27, 231–235. [Google Scholar] [CrossRef]
- White, T.C.; Marr, K.A.; Bowden, R.A. Clinical, Cellular, and Molecular Factors That Contribute to Antifungal Drug Resistance. Clin. Microbiol. Rev. 1998, 11, 382–402. [Google Scholar] [CrossRef] [Green Version]
- Sanglard, D. Emerging Threats in Antifungal-Resistant Fungal Pathogens. Front. Med. 2016, 3, 165. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, S.; Esquivel, B.D.; White, T.C. Overexpression or Deletion of Ergosterol Biosynthesis Genes Alters Doubling Time, Response to Stress Agents, and Drug Susceptibility inSaccharomyces cerevisiae. mBio 2018, 9, e01291-18. [Google Scholar] [CrossRef] [Green Version]
- Hull, C.M.; Bader, O.; Parker, J.; Weig, M.; Gross, U.; Warrilow, A.; Kelly, D.E.; Kelly, S.L. Two Clinical Isolates of Candida glabrata Exhibiting Reduced Sensitivity to Amphotericin B Both Harbor Mutations in ERG2. Antimicrob. Agents Chemother. 2012, 56, 6417–6421. [Google Scholar] [CrossRef] [Green Version]
- Mesa-Arango, A.C.; Rueda, C.; Román, E.; Quintin, J.; Terrón, M.C.; Luque, D.; Netea, M.G.; Pla, J.; Zaragoza, O. Cell Wall Changes in Amphotericin B-Resistant Strains from Candida tropicalis and Relationship with the Immune Responses Elicited by the Host. Antimicrob. Agents Chemother. 2016, 60, 2326–2335. [Google Scholar] [CrossRef] [Green Version]
- Posch, W.; Blatzer, M.; Wilflingseder, D.; Lass-Floerl, C. Aspergillus terreus: Novel lessons learned on amphotericin B resistance. Med. Mycol. 2018, 56, S73–S82. [Google Scholar] [CrossRef] [Green Version]
- Costa, C.; Ponte, A.; Pais, P.; Santos, R.; Cavalheiro, M.; Yaguchi, T.; Chibana, H.; Teixeira, M.C. New Mechanisms of Flucytosine Resistance in C. glabrata Unveiled by a Chemogenomics Analysis in S. cerevisiae. PLoS ONE 2015, 10, e0135110. [Google Scholar] [CrossRef] [PubMed]
- Gsaller, F.; Furukawa, T.; Carr, P.D.; Rash, B.; Jöchl, C.; Bertuzzi, M.; Bignell, E.; Bromley, M.J. Mechanistic Basis of pH-Dependent 5-Flucytosine Resistance inAspergillus fumigatus. Antimicrob. Agents Chemother. 2018, 62, e02593-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cowen, L.; Sanglard, D.; Howard, S.J.; Rogers, P.D.; Perlin, D. Mechanisms of Antifungal Drug Resistance. Cold Spring Harb. Perspect. Med. 2014, 5, a019752. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.F.; Rodrigues, M.E.; Henriques, M. Susceptibility of Candida glabrata biofilms to echinocandins: Alterations in the matrix composition. Biofouling 2018, 34, 569–578. [Google Scholar] [CrossRef] [Green Version]
- Perlin, D. Echinocandin Resistance in Candida. Clin. Infect. Dis. 2015, 61, S612–S617. [Google Scholar] [CrossRef] [Green Version]
- Al-Hatmi, A.M.S.; Normand, A.-C.; Ranque, S.; Piarroux, R.; De Hoog, G.S.; Meletiadis, J.; Meis, J.F. Comparative Evaluation of Etest, EUCAST, and CLSI Methods for Amphotericin B, Voriconazole, and Posaconazole against Clinically Relevant Fusarium Species. Antimicrob. Agents Chemother. 2016, 61, e01671-16. [Google Scholar] [CrossRef] [Green Version]
- Al-Hatmi, A.M.; Meis, J.F.; De Hoog, G.S. Fusarium: Molecular Diversity and Intrinsic Drug Resistance. PLOS Pathog. 2016, 12, e1005464. [Google Scholar] [CrossRef]
- Johnson, M.E.; Katiyar, S.K.; Edlind, T.D. New Fks Hot Spot for Acquired Echinocandin Resistance in Saccharomyces cerevisiae and Its Contribution to Intrinsic Resistance of Scedosporium Species. Antimicrob. Agents Chemother. 2011, 55, 3774–3781. [Google Scholar] [CrossRef] [Green Version]
- Caramalho, R.; Tyndall, J.D.A.; Monk, B.C.; Larentis, T.; Lass-Flörl, C.; Lackner, M. Intrinsic short-tailed azole resistance in mucormycetes is due to an evolutionary conserved aminoacid substitution of the lanosterol 14α-demethylase. Sci. Rep. 2017, 7, 15898. [Google Scholar] [CrossRef]
- Schell, W.A.; Jones, A.M.; Garvey, E.P.; Hoekstra, W.J.; Schotzinger, R.J.; Alexander, B.D. Fungal CYP51 Inhibitors VT-1161 and VT-1129 Exhibit Strong In Vitro Activity against Candida glabrata and C. krusei Isolates Clinically Resistant to Azole and Echinocandin Antifungal Compounds. Antimicrob. Agents Chemother. 2017, 61, e01817-16. [Google Scholar] [CrossRef] [Green Version]
- Database of Privately and Publicly Funded Clinical Studies. Available online: https://clinicaltrials.gov/ct2/home (accessed on 2 March 2020).
- VT-1161 and VT-1598 Pipeline. Available online: https://www.mycovia.com/pipeline (accessed on 2 March 2020).
- Colley, T.; Sharma, C.; Alanio, A.; Kimura, G.; Daly, L.; Nakaoki, T.; Nishimoto, Y.; Bretagne, S.; Kizawa, Y.; Strong, P.; et al. Anti-fungal activity of a novel triazole, PC1244, against emerging azole-resistant Aspergillus fumigatus and other species of Aspergillus. J. Antimicrob. Chemother. 2019, 74, 2950–2958. [Google Scholar] [CrossRef] [PubMed]
- Rauseo, A.M.; Coler-Reilly, A.; Larson, L.; Spec, A. Hope on the Horizon: Novel Fungal Treatments in Development. Open Forum Infect. Dis. 2020, 7, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiederhold, N.P. The antifungal arsenal: Alternative drugs and future targets. Int. J. Antimicrob. Agents 2017, 51, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Gintjee, T.J.; Donnelley, M.A.; Thompson, G.R. Aspiring Antifungals: Review of Current Antifungal Pipeline Developments. J. Fungi 2020, 6, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicola, A.M.; Albuquerque, P.; Paes, H.C.; Fernandes, L.; Costa, F.F.; Kioshima, E.S.; Abadio, A.K.R.; Bocca, A.L.; Felipe, M.S. Antifungal drugs: New insights in research & development. Pharmacol. Ther. 2019, 195, 21–38. [Google Scholar] [PubMed]
- Perfect, J.R. The antifungal pipeline: A reality check. Nat. Rev. Drug Discov. 2017, 16, 603–616. [Google Scholar] [CrossRef] [Green Version]
- Alkhazraji, S.; Gebremariam, T.; Alqarihi, A.; Gu, Y.; Mamouei, Z.; Singh, S.; Wiederhold, N.P.; Shaw, K.J.; Ibrahim, A.S. Fosmanogepix (APX001) Is Effective in the Treatment of Immunocompromised Mice Infected with Invasive Pulmonary Scedosporiosis or Disseminated Fusariosis. Antimicrob. Agents Chemother. 2020, 64, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Van Daele, R.; Spriet, I.; Wauters, J.; Maertens, J.; Mercier, T.; Van Hecke, S.; Brüggemann, R. Antifungal drugs: What brings the future? Med. Mycol. 2019, 57, S328–S343. [Google Scholar] [CrossRef] [Green Version]
- Su, H.; Han, L.; Huang, X. Potential targets for the development of new antifungal drugs. J. Antibiot. 2018, 71, 978–991. [Google Scholar] [CrossRef]
- Haranahalli, K.; Lazzarini, C.; Sun, Y.; Zambito, J.; Pathiranage, S.; McCarthy, J.B.; Mallamo, J.P.; Del Poeta, M.; Ojima, I. SAR Studies on Aromatic Acylhydrazone-Based Inhibitors of Fungal Sphingolipid Synthesis as Next-Generation Antifungal Agents. J. Med. Chem. 2019, 62, 8249–8273. [Google Scholar] [CrossRef]
- Lazzarini, C.; Haranahalli, K.; Rieger, R.; Ananthula, H.K.; Desai, P.B.; Ashbaugh, A.; Linke, M.J.; Cushion, M.T.; Ruzsicska, B.; Haley, J.; et al. Acylhydrazones as Antifungal Agents Targeting the Synthesis of Fungal Sphingolipids. Antimicrob. Agents Chemother. 2018, 62, e00156-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, M.; Simões, M. Advances in the antimicrobial and therapeutic potential of siderophores. Environ. Chem. Lett. 2019, 17, 1485–1494. [Google Scholar] [CrossRef]
- Lamb, A.L. Breaking a pathogen’s iron will: Inhibiting siderophore production as an antimicrobial strategy. Biochim. Biophys. Acta Proteins Proteom. 2015, 1854, 1054–1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Campo, J.S.M.; Vogelaar, N.; Tolani, K.; Kizjakina, K.; Harich, K.; Sobrado, P. Inhibition of the Flavin-Dependent Monooxygenase Siderophore A (SidA) Blocks Siderophore Biosynthesis and Aspergillus fumigatus Growth. ACS Chem. Boil. 2016, 11, 3035–3042. [Google Scholar] [CrossRef]
- Leblanc, C.; Prudhomme, T.; Tabouret, G.; Ray, A.; Burbaud, S.; Cabantous, S.; Mourey, L.; Guilhot, C.; Chalut, C. 4′-Phosphopantetheinyl Transferase PptT, a New Drug Target Required for Mycobacterium tuberculosis Growth and Persistence In Vivo. PLOS Pathog. 2012, 8, e1003097. [Google Scholar] [CrossRef] [Green Version]
- Foley, T.L.; Young, B.S.; Burkart, M.D. Phosphopantetheinyl transferase inhibition and secondary metabolism. FEBS J. 2009, 276, 7134–7145. [Google Scholar] [CrossRef]
- Golonka, R.; Vijay-Kumar, M.; Vijay-Kumar, M. The Iron Tug-of-War between Bacterial Siderophores and Innate Immunity. J. Innate Immun. 2019, 11, 249–262. [Google Scholar] [CrossRef]
- Mammen, M.P.; Armas, D.; Hughes, F.H.; Hopkins, A.M.; Fisher, C.L.; Resch, P.A.; Rusalov, D.; Sullivan, S.M.; Smith, L.R. First-in-Human Phase 1 Study To Assess Safety, Tolerability, and Pharmacokinetics of a Novel Antifungal Drug, VL-2397, in Healthy Adults. Antimicrob. Agents Chemother. 2019, 63, 1–41. [Google Scholar] [CrossRef]
- Petrik, M.; Zhai, C.; Haas, H.; Decristoforo, C. Siderophores for molecular imaging applications. Clin. Transl. Imaging 2016, 5, 15–27. [Google Scholar] [CrossRef] [Green Version]
- Luptáková, D.; Pluháček, T.; Petrik, M.; Novak, J.; Palyzová, A.; Sokolová, L.; Škríba, A.; Šedivá, B.; Lemr, K.; Havlicek, V. Non-invasive and invasive diagnoses of aspergillosis in a rat model by mass spectrometry. Sci. Rep. 2017, 7, 16523. [Google Scholar] [CrossRef] [Green Version]







| Triazole | FLC [32] | ITC [33] | VOR [34] | POS [35] | ISV [36] |
|---|---|---|---|---|---|
| Bioavailability [%] | 90 | 55 | 90–96 | variable | >98 |
| Protein binding [%] | 11–12 | 99 | 51–67 | >98 | >99 |
| Metabolism (CYP) | – | 3A4 | 2C19/2C9/3A4 | glucuronidation | 3A4/3A5 |
| Half-life [h] | 27–37 | 15–42 | 6 | 15–35 | 80–130 |
| Route of elimination | renal | hepatic | hepatic | hepatic | hepatic |
| Unchanged [%] | 64–90 | 3–18 | <2 | 66 | 45 |
| Echinocandin | ANF [38,39] | CSF [38,40] | MCF [38,41] |
|---|---|---|---|
| Bioavailability [%] | 2–7 (p.o.), 100 (iv.) | 100 (iv.) | 100 (iv.) |
| Protein binding [%] | 99 | 95 | >99 |
| Metabolism (CYP) | – | – | 3A |
| Half-life [h] | 40–50 | 8 | 13–20 |
| Route of elimination | hepatic | renal | hepatic |
| Unchanged [%] | 10 | 1.4 | <1 |
| Population | Neonatals (IC) [42] | Neonatals (IA) [43] | Children (IC) [48] | Children (IA) [43] | Adults (IC) [44] | HSCT (IA) [45] | CMTH (IA) [45] | NN (IA) [45] | NH (IA) [45] |
|---|---|---|---|---|---|---|---|---|---|
| AMBD | 0.5–1.5 | 1.0–1.5 | 0.6–1.5 | 1.0–1.5 | note 1 | note 1 | note 1 | note 1 | note 2 |
| ABCD | 3–5 | note 1 | note 1 | note 1 | note 1 | note 1 | note 1 | note 1 | note 2 |
| ABLC | 5 | 5 | 1–5 | 5 | note 1 | note 1 | note 1 | note 1 | note 2 |
| LAMB | 1–5 | 3 | 1–5 | 3 | 3 | 3 | 3 | 3 | note 1 |
| 5-FC | 100 | note 2 | 25–100 | note 2 | note 2 | note 2 | note 2 | note 2 | note 2 |
| FLC | 12 | note 2 | 6–12 | note 2 | note 1 | note 2 | note 2 | note 2 | note 2 |
| ITC | note 2 | note 3 | 5–10 | 10 | note 1 | note 2 | note 1 | note 2 | note 2 |
| VOR | note 3 | note 3 | 8–16 | 8–16 | 3–6 | note 1 | note 1 | note 1 | note 1 |
| POS | note 2 | note 3 | note 3 | note 3 | note 1 | 2–12 | 8–12 | 2–12 | note 1 |
| ISV | note 1 | note 2 | note 2 | note 2 | note 2 | note 2 | note 2 | note 2 | note 2 |
| ANF | 1.5 | note 1 | note 3 | note 1 | 100–200 4 | note 1 | note 2 | note 1 | note 2 |
| CSF | 0.5–2 | 25–70 5 | 50–70 5 | 50–70 5 | 50–70 4 | note 1 | 70 5 | note 1 | note 1 |
| MCF | 4–15 | note 3 | 2–4 | note 3 | 100 4 | note 1 | 100 4 | note 1 | note 2 |
| AA | MIC90 [mg/L] | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Aspergillus | ||||||||||
| flavus | fumigatus | nidulans | niger | terreus | ||||||
| S≤ | R> | S≤ | R> | S≤ | R> | S≤ | R> | S≤ | R> | |
| AMB | – | – | 1 | 1 | – | – | 1 | 1 | – | – |
| ANF | IE | IE | IE | IE | IE | IE | IE | IE | IE | IE |
| CSF | IE | IE | IE | IE | IE | IE | IE | IE | IE | IE |
| FLC | – | – | – | – | – | – | – | – | – | – |
| ISV | 1 | 2 | 1 | 2 | 0.25 | 0.25 | IE | IE | 1 | 1 |
| ITC | 1 | 1 | 1 | 1 | 1 | 1 | IE | IE | 1 | 1 |
| MCF | IE | IE | IE | IE | IE | IE | IE | IE | IE | IE |
| POS | IE | IE | 0.125 | 0.25 | IE | IE | IE | IE | 0.125 | 0.25 |
| VOR | IE | IE | 1 | 1 | 1 | 1 | IE | IE | IE | IE |
| AA | MIC90 [mg/L] | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Candida | ||||||||||||
| albicans | dubliniensis | glabrata | krusei | parapsilosis | tropicalis | |||||||
| S≤ | R> | S≤ | R> | S≤ | R> | S≤ | R> | S≤ | R> | S≤ | R> | |
| AMB | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| ANF | 0.03 | 0.03 | – | – | 0.06 | 0.06 | 0.06 | 0.06 | 4 | 4 | 0.06 | 0.06 |
| CSF 1 | – | – | – | – | – | – | – | – | – | – | – | – |
| FLC | 2 | 4 | 2 | 4 | 0.001 | 16 | IE | IE | 2 | 4 | 2 | 4 |
| ISV | IE | IE | IE | IE | IE | IE | IE | IE | IE | IE | IE | IE |
| ITC | 0.06 | 0.06 | 0.06 | 0.06 | IE | IE | IE | IE | 0.125 | 0.125 | 0.125 | 0.125 |
| MCF | 0.016 | 0.016 | – | – | 0.03 | 0.03 | IE | IE | 2 | 2 | IE | IE |
| POS | 0.06 | 0.06 | 0.06 | 0.06 | IE | IE | IE | IE | 0.06 | 0.06 | 0.06 | 0.06 |
| VOR | 0.06 | 0.25 | 0.06 | 0.25 | IE | IE | IE | IE | 0.125 | 0.25 | 0.125 | 0.25 |
| AA | Identification | Last Update | Phase 0 | Phase 1 | Phase 2 | Phase 3 | Phase 4 |
|---|---|---|---|---|---|---|---|
| VT-1129 [79] | – | – | |||||
| VT-1161 [80] | NCT03562156 | 5th Feb 2020 | |||||
| VT-1598 [81] | – | – | |||||
| PC1244 [82] | – | – | |||||
| SUBA-ITC [80] | NCT03572049 | 25th Oct 2019 | |||||
| CAMB [80] | NCT02971007 | 2nd Nov 2018 | |||||
| Rezafungin [80] | NCT03667690 | 5th Feb 2020 | |||||
| Ibrexafungerp [80] | NCT03363841 | 2nd Dec 2019 | |||||
| Fosmanogepix [80] | NCT03604705 | 28th Feb 2020 | |||||
| Olorofim [80] | NCT03583164 | 18th Jan 2020 | |||||
| MGCD290 [80] | NCT01497223 | 4th April 2013 | |||||
| T-2307 [83] | – | – | |||||
| VL-2397 [80] | NCT03327727 | 27th Feb 2019 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Houšť, J.; Spížek, J.; Havlíček, V. Antifungal Drugs. Metabolites 2020, 10, 106. https://doi.org/10.3390/metabo10030106
Houšť J, Spížek J, Havlíček V. Antifungal Drugs. Metabolites. 2020; 10(3):106. https://doi.org/10.3390/metabo10030106
Chicago/Turabian StyleHoušť, Jiří, Jaroslav Spížek, and Vladimír Havlíček. 2020. "Antifungal Drugs" Metabolites 10, no. 3: 106. https://doi.org/10.3390/metabo10030106
APA StyleHoušť, J., Spížek, J., & Havlíček, V. (2020). Antifungal Drugs. Metabolites, 10(3), 106. https://doi.org/10.3390/metabo10030106