Integrative Metabolomics for Assessing the Effect of Insect (Hermetia illucens) Protein Extract on Rainbow Trout Metabolism
Abstract
:1. Introduction
2. Results
2.1. Fish Growth
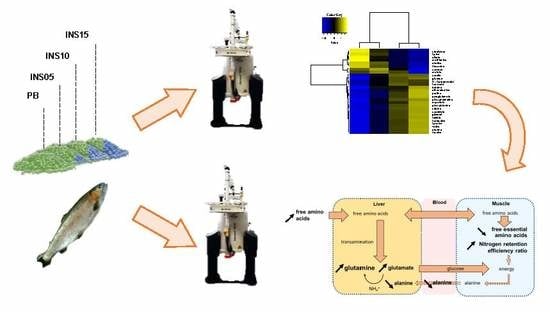
2.2. Annotation and Integration of Feed Soluble Compounds and Fish Metabolome NMR Spectra
2.3. Patterns of Compounds in Feed
2.4. Fish Separation Based on Insect Amount in Diet
3. Discussion
3.1. Protein Synthesis-Oriented Metabolism Induced by Dietary Compounds
3.2. Metabolites as Markers of Alteration of Metabolism Induced by PB Diet
4. Materials and Methods
4.1. Growth Trial and Sampling
4.2. Diet and Fish Whole Body Composition Analysis.
4.3. Sample preparation
4.4. Total Amino Acids in Feeds
4.5. NMR Acquisition
4.6. NMR Spectra Processing
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO. La Situation Mondiale des Pêches et de l’aquaculture 2018. In Atteindre Les Objectifs de Développement Durable; FAO: Rome, Italy, 2018; ISBN 978-92-5-130692-5. [Google Scholar]
- Francis, G.; Makkar, H.P.S.; Becker, K. Antinutritional factors present in plant-derived alternate fish feed ingredients and their effects in fish. Aquaculture 2001, 199, 197–227. [Google Scholar] [CrossRef]
- Tacon, A.G.J.; Hasan, M.R.; Métian, M. Demand and Supply of Feed Ingredients for Farmed Fish and Crustaceans. In Trends and Prospects; Food and Agriculture Organization of the United Nations: Rome, Italy, 2011; ISBN 978-92-5-106933-2. [Google Scholar]
- Magalhães, R.; Sánchez-López, A.; Leal, R.S.; Martínez-Llorens, S.; Oliva-Teles, A.; Peres, H. Black soldier fly (Hermetia illucens) pre-pupae meal as a fish meal replacement in diets for European seabass (Dicentrarchus labrax). Aquaculture 2017, 476, 79–85. [Google Scholar] [CrossRef]
- Stadtlander, T.; Stamer, A.; Buser, A.; Wohlfahrt, J.; Leiber, F.; Sandrock, C. Hermetia illucens meal as fish meal replacement for rainbow trout on farm. J. Insects Food Feed 2017, 3, 165–175. [Google Scholar] [CrossRef] [Green Version]
- Lock, E.R.; Arsiwalla, T.; Waagbø, R. Insect larvae meal as an alternative source of nutrients in the diet of Atlantic salmon (Salmo salar) postsmolt. Aquac. Nutr. 2016, 22, 1202–1213. [Google Scholar] [CrossRef]
- St-Hilaire, S.; Sheppard, C.; Tomberlin, J.K.; Irving, S.; Newton, L.; McGuire, M.A.; Mosley, E.E.; Hardy, R.W.; Sealey, W. Fly prepupae as a feedstuff for rainbow trout, Oncorhynchus mykiss. J. World. Aquac. Soc. 2007, 38, 59–67. [Google Scholar] [CrossRef]
- Barragan-Fonseca, K.B.; Dicke, M.; van Loon, J.J.A. Nutritional value of the black soldier fly (Hermetia illucens L.) and its suitability as animal feed—A review. J. Insects Food Feed 2017, 3, 105–120. [Google Scholar] [CrossRef]
- Henry, M.; Gasco, L.; Piccolo, G.; Fountoulaki, E. Review on the use of insects in the diet of farmed fish: Past and future. Anim. Feed Sci. Technol. 2015, 203, 1–22. [Google Scholar] [CrossRef]
- Aksnes, A.; Hope, B.; Jönsson, E.; Björnsson, B.T.; Albrektsen, S. Size-fractionated fish hydrolysate as feed ingredient for rainbow trout (Oncorhynchus mykiss) fed high plant protein diets. I: Growth, growth regulation and feed utilization. Aquaculture 2006, 261, 305–317. [Google Scholar] [CrossRef]
- Wei, Y.; Liang, M.; Mai, K.; Zheng, K.; Xu, H. 1H NMR-based metabolomics studies on the effect of size-fractionated fish protein hydrolysate, fish meal and plant protein in diet for juvenile turbot (Scophthalmus maximus L.). Aquac. Nutr. 2017, 23, 523–536. [Google Scholar] [CrossRef]
- Refstie, S.; Olli, J.J.; Standal, H. Feed intake, growth, and protein utilisation by post-smolt Atlantic salmon (Salmo salar) in response to graded levels of fish protein hydrolysate in the diet. Aquaculture 2004, 239, 331–349. [Google Scholar] [CrossRef]
- Hou, Y.; Wu, Z.; Dai, Z.; Wang, G.; Wu, G. Protein hydrolysates in animal nutrition: Industrial production, bioactive peptides, and functional significance. J. Anim. Sci. Biotechnol. 2017, 8, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Yan, X.; Lu, R.; Meng, X.; Nie, G. Peptide transporter 1 (PepT1) in fish: A review. Aquac. Fish. 2017, 2, 193–206. [Google Scholar] [CrossRef]
- Verri, T.; Terova, G.; Dabrowski, K.; Saroglia, M. Peptide transport and animal growth: The fish paradigm. Biol. Lett. 2011, 7, 597–600. [Google Scholar] [CrossRef] [PubMed]
- Glencross, B.D.; Booth, M.; Allan, G.L. A feed is only as good as its ingredients: A review of ingredient evaluation strategies for aquaculture feeds. Aquac. Nutr. 2007, 13, 17–34. [Google Scholar] [CrossRef]
- Geay, F.; Ferraresso, S.; Zambonino-Infante, J.L.; Bargelloni, L.; Quentel, C.; Vandeputte, M.; Kaushik, S.; Cahu, C.L.; Mazurais, D. Effects of the total replacement of fish-based diet with plant-based diet on the hepatic transcriptome of two European sea bass (Dicentrarchus labrax) half-sibfamilies showing different growth rates with the plant-based diet. BMC Genomics 2011, 12, 522. [Google Scholar] [CrossRef] [Green Version]
- Panserat, S.; Hortopan, G.A.; Plagnes-Juan, E.; Kolditz, C.; Lansard, M.; Skiba-Cassy, S.; Esquerré, D.; Geurden, I.; Médale, F.; Kaushik, S.; et al. Differential gene expression after total replacement of dietary fish meal and fish oil by plant products in rainbow trout (Oncorhynchus mykiss) liver. Aquaculture 2009, 294, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Casu, F.; Watson, A.M.; Yost, J.; Leffler, J.W.; Gaylord, T.G.; Barrows, F.T.; Sandifer, P.A.; Denson, M.R.; Bearden, D.W. Investigation of graded-level soybean meal diets in red drum (Sciaenops ocellatus) using NMR-based metabolomics analysis. Comp. Biochem. Physiol. Part D Genom. Proteom. 2018, 29, 173–184. [Google Scholar] [CrossRef]
- Schock, T.B.; Newton, S.; Brenkert, K.; Leffler, J.; Bearden, D.W. An NMR-based metabolomic assessment of cultured cobia health in response to dietary manipulation. Food Chem. 2012, 133, 90–101. [Google Scholar] [CrossRef]
- Abro, R.; Moazzami, A.A.; Lindberg, J.E.; Lundh, T. Metabolic insights in Arctic charr (Salvelinus alpinus) fed with zygomycetes and fish meal diets as assessed in liver using nuclear magnetic resonance (NMR) spectroscopy. Int. Aquat. Res. 2014, 6, 6–63. [Google Scholar] [CrossRef] [Green Version]
- Gatesoupe, F.-J.; Fauconneau, B.; Deborde, C.; Madji Hounoum, B.; Jacob, D.; Moing, A.; Corraze, G.; Médale, F. Intestinal microbiota in rainbow trout, Oncorhynchus mykiss, fed diets with different levels of fish-based and plant ingredients: A correlative approach with some plasma metabolites. Aquac. Nutr. 2018, 24, 1563–1576. [Google Scholar] [CrossRef]
- Asakura, T.; Sakata, K.; Yoshida, S.; Date, Y.; Kikuchi, J. Noninvasive analysis of metabolic changes following nutrient input into diverse fish species, as investigated by metabolic and microbial profiling approaches. PeerJ 2014, 2, e550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mekuchi, M.; Sakata, K.; Yamaguchi, T.; Koiso, M.; Kikuchi, J. Trans-omics approaches used to characterise fish nutritional biorhythms in leopard coral grouper (Plectropomus leopardus). Sci. Rep. 2017, 7, 9372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roques, S.; Deborde, C.; Richard, N.; Sergent, L.; Kurz, F.; Skiba-Cassy, S.; Fauconneau, B.; Moing, A. Characterizing alternative feeds for rainbow trout (O. mykiss) by 1H NMR metabolomics. Metabolomics 2018, 14, 305. [Google Scholar] [CrossRef] [Green Version]
- Jasour, M.S.; Wagner, L.; Sundekilde, U.K.; Larsen, B.K.; Rasmussen, H.T.; Hjermitslev, N.H.; Hammershøj, M.; Dalsgaard, A.J.T.; Dalsgaard, T.K. Fishmeal with different levels of biogenic amines in aquafeed: Comparison of feed protein quality, fish growth performance, and metabolism. Aquaculture 2018, 488, 80–89. [Google Scholar] [CrossRef]
- De la Higuera, M. Effects of Nutritional Factors and Feed Characteristics on Feed Intake. In Food Intake in Fish; Houlihan, D., Boujard, T., Jobling, M., Eds.; Blackwell Science: Oxford, UK, 2001; pp. 250–268. [Google Scholar]
- Kaushik, S.J.; Seiliez, I. Protein and amino acid nutrition and metabolism in fish: Current knowledge and future needs. Aquac. Res. 2010, 41, 322–332. [Google Scholar] [CrossRef]
- Wright, P.A. Nitrogen excretion: Three end products, many physiological roles. J. Exp. Biol. 1995, 198, 273–281. [Google Scholar]
- Ip, Y.K.; Chew, S.F. Ammonia Production, Excretion, Toxicity, and Defense in Fish: A Review. Front. Physiol. 2010, 1, 134. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, T.; Unuma, T.; Akiyama, T. Postprandial changes in plasma free amino acid concentrations of rainbow trout fed diets containing different protein sources. Fish. Sci. 1998, 64, 474–481. [Google Scholar] [CrossRef] [Green Version]
- Salze, G.P.; Davis, D.A. Taurine: A critical nutrient for future fish feeds. Aquaculture 2015, 437, 215–229. [Google Scholar] [CrossRef]
- Yokoyama, M.; Nakazoe, J.-I. Accumulation and excretion of taurine in rainbow trout (Oncorhynchus mykiss) fed diets supplemented with methionine, cystine and taurine. Comp. Biochem. Physiol. A Physiol. 1992, 102, 565–568. [Google Scholar] [CrossRef]
- Kataoka, H.; Ohnishi, N. Occurrence of Taurine in Plants. Agric. Biol. Chem. 2014, 50, 1887–1888. [Google Scholar] [CrossRef]
- Belghit, I.; Liland, N.S.; Gjesdal, P.; Biancarosa, I.; Menchetti, E.; Li, Y.; Waagbø, R.; Krogdahl, Å.; Lock, E.-J. Black soldier fly larvae meal can replace fish meal in diets of sea-water phase Atlantic salmon (Salmo salar). Aquaculture 2019, 503, 609–619. [Google Scholar] [CrossRef]
- Krogdahl, Å.; Penn, M.; Thorsen, J.; Refstie, S.; Bakke, A.M. Important antinutrients in plant feedstuffs for aquaculture: An update on recent findings regarding responses in salmonids. Aquac. Res. 2010, 41, 333–344. [Google Scholar] [CrossRef]
- Gómez-Requeni, P.; Mingarro, M.; Calduch-Giner, J.A.; Médale, F.; Martin, S.A.M.; Houlihan, D.F.; Kaushik, S.; Pérez-Sánchez, J. Protein growth performance, amino acid utilisation and somatotropic axis responsiveness to fish meal replacement by plant protein sources in gilthead sea bream (Sparus aurata). Aquaculture 2004, 232, 493–510. [Google Scholar] [CrossRef]
- Lin, C.Y.; Wu, H.; Tjeerdema, R.S.; Viant, M.R. Evaluation of metabolite extraction strategies from tissue samples using NMR metabolomics. Metabolomics 2007, 3, 55–67. [Google Scholar] [CrossRef]
- Jacob, D.; Deborde, C.; Lefebvre, M.; Maucourt, M.; Moing, A. NMRProcFlow: A graphical and interactive tool dedicated to 1D spectra processing for NMR-based metabolomics. Metabolomics 2017, 13, 36. [Google Scholar] [CrossRef] [Green Version]
- Kullgren, A.; Samuelsson, L.M.; Larsson, D.G.J.; Bjornsson, B.T.; Bergman, E.J. A metabolomics approach to elucidate effects of food deprivation in juvenile rainbow trout (Oncorhynchus mykiss). Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R1440–R1448. [Google Scholar] [CrossRef] [Green Version]
- Shen, G.; Huang, Y.; Dong, J.; Wang, X.; Cheng, K.-K.; Feng, J.; Xu, J.; Ye, J. Metabolic effect of dietary taurine supplementation on Nile tilapia (Oreochromis nilotictus) evaluated by NMR-based Metabolomics. J. Agric. Food Chem. 2018, 66, 368–377. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.-M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef] [Green Version]





| Zootechnical Parameters | PB | INS05 | INS10 | INS15 |
|---|---|---|---|---|
| Initial body weight (g) † | 48.67 ± 1.35 | 48.90 ± 1.01 | 48.90 ± 1.01 | 48.90 ± 1.01 |
| Final body weight (g) † | 247.33 ± 13.99 a * | 258.67 ± 11.57 a | 293.56 ± 5.35 b | 303.33 ± 8.33 b |
| Daily feed intake (% of body weight per day) † | 1.57 ± 0.03 a | 1.50 ± 0.03 ab | 1.51 ± 0.03 ab | 1.48 ± 0.03 b |
| Feed conversion ratio† | 0.98 ± 0.03 a | 0.92 ± 0.03 ab | 0.89 ± 0.02 bc | 0.86 ± 0.02 c |
| Nitrogen efficiency ratio (N retention % of N intake) † | 40.32 ± 1.55 a | 44.68 ± 1.58 ab | 46.47 ± 2.48 b | 48.66 ± 1.06 b |
| Hepatosomatic index ‡ | 0.77 ± 0.12 | 0.87 ± 0.11 | 0.84 ± 0.19 | 0.84 ± 0.9 |
| Viscerosomatic index ‡ | 8.96 ± 1.37 | 9.77 ± 1.34 | 9.09 ± 0.58 | 8.74 ± 0.85 |
| Ingredients | PB | INS05 | INS10 | INS15 |
|---|---|---|---|---|
| DHA*-rich algae meal | 6.84 | 6.84 | 6.84 | 6.84 |
| Insect protein hydrolysate | 5.00 | 10.00 | 15.00 | |
| Vegetable oils (a) | 18.10 | 16.95 | 16.00 | 14.95 |
| Plant proteins (b) | 70.40 | 66.74 | 62.78 | 58.79 |
| Rapeseed lecithin | 1.00 | 1.00 | 1.00 | 1.00 |
| Monocalcium phosphate | 1.20 | 1.10 | 1.00 | 1.00 |
| Phytase | 0.02 | 0.02 | 0.02 | 0.02 |
| Lysine 78% | 0.50 | 0.50 | 0.50 | 0.50 |
| DL-methionine 98% | 0.65 | 0.56 | 0.57 | 0.61 |
| Threonine 98% | 0.20 | 0.20 | 0.20 | 0.20 |
| Vitamin premix | 0.30 | 0.30 | 0.30 | 0.30 |
| Vitamin C monophosphate 35 | 0.04 | 0.04 | 0.04 | 0.04 |
| Mineral premix | 0.30 | 0.30 | 0.30 | 0.30 |
| Liquid choline | 0.15 | 0.15 | 0.15 | 0.15 |
| Antioxidant | 0.15 | 0.15 | 0.15 | 0.15 |
| Antifungal | 0.15 | 0.15 | 0.15 | 0.15 |
| Energy (kJ.g−1 dry matter) | 24.03 | 24.50 | 24.59 | 24.71 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roques, S.; Deborde, C.; Guimas, L.; Marchand, Y.; Richard, N.; Jacob, D.; Skiba-Cassy, S.; Moing, A.; Fauconneau, B. Integrative Metabolomics for Assessing the Effect of Insect (Hermetia illucens) Protein Extract on Rainbow Trout Metabolism. Metabolites 2020, 10, 83. https://doi.org/10.3390/metabo10030083
Roques S, Deborde C, Guimas L, Marchand Y, Richard N, Jacob D, Skiba-Cassy S, Moing A, Fauconneau B. Integrative Metabolomics for Assessing the Effect of Insect (Hermetia illucens) Protein Extract on Rainbow Trout Metabolism. Metabolites. 2020; 10(3):83. https://doi.org/10.3390/metabo10030083
Chicago/Turabian StyleRoques, Simon, Catherine Deborde, Laurence Guimas, Yann Marchand, Nadège Richard, Daniel Jacob, Sandrine Skiba-Cassy, Annick Moing, and Benoit Fauconneau. 2020. "Integrative Metabolomics for Assessing the Effect of Insect (Hermetia illucens) Protein Extract on Rainbow Trout Metabolism" Metabolites 10, no. 3: 83. https://doi.org/10.3390/metabo10030083
APA StyleRoques, S., Deborde, C., Guimas, L., Marchand, Y., Richard, N., Jacob, D., Skiba-Cassy, S., Moing, A., & Fauconneau, B. (2020). Integrative Metabolomics for Assessing the Effect of Insect (Hermetia illucens) Protein Extract on Rainbow Trout Metabolism. Metabolites, 10(3), 83. https://doi.org/10.3390/metabo10030083