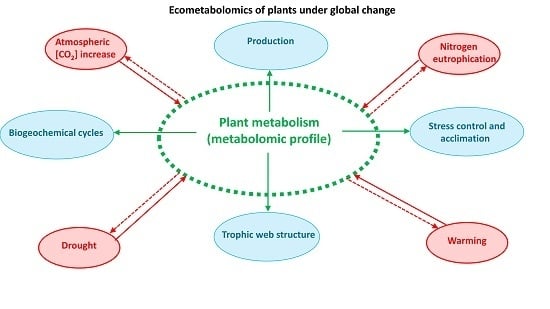
Ecometabolomics for a Better Understanding of Plant Responses and Acclimation to Abiotic Factors Linked to Global Change
Abstract
:1. Background
2. Drought
3. Heat Stress
4. Elevated Atmospheric CO2 Concentrations
5. Nitrogen Eutrophication
6. Concluding Remarks
7. Material and Methods
Data Collection
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Penuelas, J.; Sardans, J. Ecological metabolomics. Chem. Ecol. 2009, 25, 305–309. [Google Scholar] [CrossRef]
- Kucina, V.; Ekstron, C.T.; Anderson, S.B.; Nielsen, J.K.; Olsen, C.E.; Bak, S. Identification of defense compounds in Barberea vulgaris against the Herbivore Phyllotreta nemorum by an ecometabolomic approach. Plant Physiol. 2009, 151, 1977–1990. [Google Scholar] [CrossRef] [Green Version]
- Sardans, J.; Peñuelas, J.; Rivas-Ubach, A. Ecological metabolomics as a proxy for organisms, populations, and species lifestyle: Current development and future challenges. Chemoecology 2011, 21, 191–225. [Google Scholar] [CrossRef]
- Rivas-Ubach, A.; Pérez-Trujillo, M.; Sardans, J.; Gargallo-Garriga, A.; Parella, T.; Penuelas, J. Ecometabolomics: Optimized NMR-based method. Methods Ecol. Evol. 2013, 4, 464–473. [Google Scholar] [CrossRef]
- Rivas-Ubach, A.; Peñuelas, J.; Hódar, J.A.; Oravec, M.; Tolic, L.P.; Urban, O.; Sardans, J. We Are What We Eat: A Stoichiometric and Ecometabolomic Study of Caterpillars Feeding on Two Pine Subspecies of Pinus sylvestris. Int. J. Mol. Sci. 2018, 20, 59. [Google Scholar] [CrossRef] [Green Version]
- Allevato, D.M.; Kiyota, E.; Mazzafera, P.; Nixon, K.C. Ecometabolomic Analysis of Wild Populations of Pilocarpus pennatifolius (Rutaceae) Using Unimodal Analyses. Front. Plant Sci. 2019, 10, 258. [Google Scholar] [CrossRef] [Green Version]
- Ozawa, R.; Shiojiri, K.; Sabelis, M.W.; Takabayashi, J. Maize plants sprayed with either jasmonic acid or its precursor, methyl linolenate, attract armyworm parasitoids, but the composition of attractants differs. Èntomol. Exp. Appl. 2008, 129, 189–199. [Google Scholar] [CrossRef]
- Llusià, J.; Penuelas, J.; Sardans, J.; Owen, S.M. Niinemets, Ülo Measurement of volatile terpene emissions in 70 dominant vascular plant species in Hawaii: Aliens emit more than natives. Glob. Ecol. Biogeogr. 2010, 19, 863–874. [Google Scholar] [CrossRef]
- Gullberg, J.; Jonsson, P.; Nordstrom, A.; Sjöström, M.; Moritz, T. Design of experiments: An efficient strategy to identify factors influencing extraction and derivatization of Arabidopsis thaliana samples in metabolomic studies with gas chromatography/mass spectrometry. Anal. Biochem. 2004, 331, 283–295. [Google Scholar] [CrossRef]
- Allwood, J.W.; Goodacre, R. An introduction to liquid chromatographya mass spectrometry instrumentation applied in plant metabolomic analyses. Phytochem. Anal. 2010, 21, 33–47. [Google Scholar] [CrossRef]
- Jennings, K.R. The changing impact of the collision-induced decomposition of ions on mass spectrometry. Int. J. Mass Spectrom. 2000, 200, 479–493. [Google Scholar] [CrossRef]
- Emwas, A.-H.; Roy, R.; McKay, R.; Tenori, L.; Saccenti, E.; Gowda, G.A.N.; Raftery, D.; AlAhmari, F.; Jaremko, Ł.; Jaremko, M.; et al. NMR Spectroscopy for Metabolomics Research. Metabolites 2019, 9, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sousa Silva, M.; Cordeiro, C.; Roessner, U.; Figuereido, A. Editorial: Metabolomics in crop research-current and emerging methodologies. Front. Plant Sci. 2019, 10, 1013. [Google Scholar] [CrossRef] [PubMed]
- Lewis, I.A.; Schommer, S.C.; Hodis, B.; Robb, K.A.; Tonelli, M.; Westler, W.M.; Sussman, M.R.; Markley, J.L. Method for Determining Molar Concentrations of Metabolites in Complex Solutions from Two-Dimensional1H−13C NMR Spectra. Anal. Chem. 2007, 79, 9385–9390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viant, M.R.; Bearden, D.W.; Bundy, J.G.; Burton, I.W.; Collette, T.W.; Ekman, E.R.; Ezernieks, V.; Karakach, T.; Lin, C.-Y.; Rochfort, S.; et al. International NMR-Based Environmental Metabolomics Intercomparison Exercise. Environ. Sci. Technol. 2009, 43, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Khakimov, B.; Bak, S.; Engelsen, S.B. High-throughput cereal metabolomics: Current analytical technologies, challenges, and perspectives. J. Cereal Sci. 2014, 59, 393–418. [Google Scholar] [CrossRef]
- Zhuang, J.; Zhang, J.; Hou, X.; Wang, F.; Xiong, F. Transcriptomic, Proteomic, Metabolomic and Functional Genomic Approaches for the Study of Abiotic Stress in Vegetable Crops. Crit. Rev. Plant Sci. 2014, 33, 225–237. [Google Scholar] [CrossRef]
- Aliferis, K.A.; Chrysayi-Takousbalides, M. Metabolomics in pesticide research and development: Review and future perspectives. Metabolomics 2011, 7, 35–53. [Google Scholar] [CrossRef]
- Kumar, M.; Kuzhiumparambil, U.; Pernice, M.; Jiang, Z.; Ralph, P. Metabolomics: An emerging frontier of systems biology in marine macrophytes. Algal Res. 2016, 16, 76–92. [Google Scholar] [CrossRef]
- Tugizimana, F.; Mhlongo, M.I.; Piater, L.A.; Dubery, I.A. Metabolomics in Plant Priming Research: The Way Forward? Int. J. Mol. Sci. 2018, 19, 1759. [Google Scholar] [CrossRef] [Green Version]
- Kumari, A.; Das, P.; Parida, A.K.; Agarwal, P.K. Proteomics, metabolomics, and ionomics perspectives of salinity tolerance in halophytes. Front. Plant Sci. 2015, 6, 537. [Google Scholar] [CrossRef] [Green Version]
- Gandhi, S.; Khushu, S.; Tripathi, R.P. Current metabolomic methodologies and their application to thermal stress. Curr. Metabol. 2013, 1, 335–352. [Google Scholar] [CrossRef]
- Jones, O.A.; Dias, D.A.; Callahan, D.; Kouremenos, K.A.; Beale, D.J.; Roessner, U. The use of metabolomics in the study of metals in biological systems. Metallomics 2015, 7, 29–38. [Google Scholar] [CrossRef]
- Paudel, J.R.; Amirizian, A.; Krosse, S.; Giddings, J.; Ismail, S.A.A.; Xia, J.; Gloer, J.B.; van Dam, N.M.; Bede, J.C. Effect of atmopspheric carbon dioxide levels and nitrate fertilization on glucosinolate biosynthesis in mechanically damaged Arabidopsis plants. BMC Plant Biol. 2016, 16, 68. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Peuke, A.D.; Zhao, X.; Yan, J.; Li, C. Effects of simulated atmospheric nitrogen deposition on foliar chemistry and physiology of hybrid poplar seedlings. Plant Physiol. Biochem. 2019, 143, 94–108. [Google Scholar] [CrossRef]
- De Souza, A.P.; Cocuron, J.-C.; Garcia, A.C.; Alonso, A.P.; Buckeridge, M.S. Changes in whole-plant metabolism during the grain-filling stage in sorghum grown under elevated CO2 and drought. Plant Physiol. 2015, 169, 1755–1765. [Google Scholar] [CrossRef]
- Austen, N.; Walker, H.J.; Lake, J.A.; Phoenix, G.K.; Cameron, D.D. The Regulation of Plant Secondary Metabolism in Response to Abiotic Stress: Interactions Between Heat Shock and Elevated CO2. Front. Plant Sci. 2019, 10, 1463. [Google Scholar] [CrossRef] [Green Version]
- Feng, S.; Fu, Q. Expansion of global drylands under a warming climate. Atmos. Chem. Phys. Discuss. 2013, 13, 10081–10094. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Yu, H.; Guan, X.; Wang, G.; Guo, R. Accelerated dryland expansion under climate change. Nat. Clim. Chang. 2015, 6, 166–171. [Google Scholar] [CrossRef]
- Rivas-Ubach, A.; Sardans, J.; Pérez-Trujillo, M.; Estiarte, M.; Peñuelas, J. Strong relationship between elemental sotichiometry and metabolome in plants. Proc. Nat. Acad. Sci. USA 2012, 109, 4181–4186. [Google Scholar] [CrossRef] [Green Version]
- Rivas-Ubach, A.; Sardans, J.; Gargallo-Garriga, A.; Parella, T.; Perez-Trujillo, M.; Estiarte, M.; Penuelas, J. Drought stress enhances folivory by shifting foliar metabolomes in Quercus ilex trees. New Phytol. 2014, 27, 874–885. [Google Scholar] [CrossRef] [Green Version]
- Ullah, N.; Yüce, M.; Gökçe, Z.N.O.; Budak, H. Comparative metabolite profiling of drought stress in roots and leaves of seven Triticeae species. BMC Genom. 2017, 18, 969. [Google Scholar] [CrossRef]
- Shahbazy, M.; Moradi, P.; Ertaylan, G.; Zahraei, A.; Kompany-Zareh, M. FTICR mass spectrometry-based multivariate analysis to explore distinctive metabolites and metabolic pathways: A comprehensive bioanalytical strategy toward time-course metabolic profiling of Thymus vulgaris plants responding to drought stress. Plant Sci. 2020, 290, 110257. [Google Scholar] [CrossRef]
- Bianco, R.L.; Rieger, M.; Sung, S.-J.S. Effect of drought on sorbitol and sucrose metabolism in sinks and sources of peach. Physiol. Plant. 2000, 108, 71–78. [Google Scholar] [CrossRef]
- Chaves, M.M.; Marôco, J.; Pereira, J.; Chaves, M.M. Understanding plant responses to drought from genes to the whole plant. Funct. Plant Boil. 2003, 30, 239–264. [Google Scholar] [CrossRef]
- Shen, B.; Jensen, R.G.; Bohnert, H.J. Mannitol Protects against Oxidation by Hydroxyl Radicals. Plant Physiol. 1997, 115, 527–532. [Google Scholar] [CrossRef] [Green Version]
- Llanes, A.; Andrade, A.; Alemano, S.; Luna, V. Metabolomic Approach to Understand Plant Adaptations to Water and Salt Stress. Plant Metab. Regul. Under Env. Stress 2018, 133–144. [Google Scholar] [CrossRef]
- Keunen, E.; Peshev, D.; Vangronsveld, J.; Ende, W.V.D.; Cuypers, A. Plant sugars are crucial players in the oxidative challenge during abiotic stress: Extending the traditional concept. Plant Cell Environ. 2013, 36, 1242–1255. [Google Scholar] [CrossRef]
- Alvarez, S.; Marsh, E.L.; Schroeder, S.G.; Schachtman, D. Metabolomic and proteomic changes in the xylem sap of maize under drought. Plant Cell Environ. 2008, 31, 325–340. [Google Scholar] [CrossRef]
- Barchet, G.L.; Dauwe, R.; Guy, R.D.; Schroeder, W.; Soolanayakanahally, R.Y.; Campbell, M.M.; Mansfield, S.D. Investigating the drought-stress response of hybrid poplar genotypes by metabolite profiling. Tree Physiol. 2013, 34, 1203–1219. [Google Scholar] [CrossRef] [Green Version]
- Gargallo-Garriga, A.; Preece, C.; Sardans, J.; Oravec, M.; Urban, O.; Penuelas, J. Root exudate metabolomes change under drought and show limited capacity for recovery. Sci. Rep. 2018, 8, 12696. [Google Scholar] [CrossRef] [Green Version]
- Nakabayashi, R.; Mori, T.; Saito, K. Alteration of flavonoid accumulation under drought stress in Arabidopsis thaliana. Plant Signal. Behav. 2014, 9, e29518. [Google Scholar] [CrossRef] [Green Version]
- Pavli, O.I.; Vlachos, C.E.; Kalloniati, C.; Flemetakis, E.; Skaracis, G.N. Metabolite profiling reveals the effect of drought on sorghum (Sorghum bicolor L. Moench) metabolism. Plant Omics J. 2013, 6, 371–376. [Google Scholar]
- Hsiao, T.C. Plant responses to water stress. Ann. Rev. Plant Physiol. 1973, 24, 519–570. [Google Scholar] [CrossRef]
- Fathi, A.; Tari, D.B. Effect of Drought Stress, and its Mechanism in Plants. Int. J. Life Sci. 2016, 10, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Ahanger, M.A.; Gul, F.; Ahmad, P.; Akram, N.A. Environmental Stresses and Metabolomics—Deciphering the Role of Stress Responsive Metabolites. In Plant Metabolites and Regulation Under Environmental Stress; Elsevier BV: Amsterdam, The Netherlands, 2018; pp. 53–67. [Google Scholar]
- Thompson, J.; Stewart, C.R.; Morris, C.J. Changes in Amino Acid Content of Excised Leaves During Incubation I. The Effect of Water Content of Leaves and Atmospheric Oxygen Level. Plant Physiol. 1966, 41, 1578–1584. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Murzello, C.; Sun, Y.; Kim, M.-S.; Xie, X.; Jeter, R.M.; Zak, J.C.; Dowd, S.E.; Pare, P.W. Choline and Osmotic-Stress Tolerance Induced in Arabidopsis by the Soil Microbe Bacillus subtilis (GB03). Mol. Plant Microbe Interact. 2010, 23, 1097–1104. [Google Scholar] [CrossRef] [Green Version]
- Gou, W.; Tian, L.; Ruan, Z.; Zheng, P.; Chen, F.; Zhang, J.; Cui, Z.; Li, Z.; Gao, M.; Shi, W.; et al. Accumulation of choline and glycinebetaine and drought stress tolerance induced in maize (Zea mays) by three plant growth promoting rhizobacteria (PGPR) strains. Pak. J. Bot. 2015, 47, 581–586. [Google Scholar]
- Hayat, S.; Hayat, Q.; Alyemeni, M.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.H.H.; Murata, N. Enhancement of tolerance of abiotic stress by metabolic engineering of betaines and other compatible solutes. Curr. Opin. Plant Boil. 2002, 5, 250–257. [Google Scholar] [CrossRef]
- Hamilton, E.W.; Heckathorn, S.A. Mitochondrial Adaptations to NaCl. Complex I Is Protected by Antioxidants and Small Heat Shock Proteins, Whereas Complex II Is Protected by Proline and Betaine1. Plant Physiol. 2001, 126, 1266–1274. [Google Scholar] [CrossRef] [Green Version]
- Perlikowski, D.; Czyżniejewski, M.; Marczak, L.; Augustyniak, A.; Kosmala, A. Water Deficit Affects Primary Metabolism Differently in Two Lolium multiflorum/Festuca arundinacea Introgression Forms with a Distinct Capacity for Photosynthesis and Membrane Regeneration. Front. Plant Sci. 2016, 7, 17. [Google Scholar] [CrossRef] [Green Version]
- Michaletti, A.; Naghavi, M.R.; Toorchi, M.; Zolla, L.; Rinalducci, S. Metabolomics and proteomics reveal drought-stress responses of leaf tissues from spring-wheat. Sci. Rep. 2018, 8, 5710. [Google Scholar] [CrossRef] [Green Version]
- Mekonnen, D.W.; Flügge, U.-I.; Ludewig, F. Gamma-aminobutyric acid depletion affects stomata closure and drought tolerance of Arabidopsis thaliana. Plant Sci. 2016, 245, 25–34. [Google Scholar] [CrossRef]
- Klem, K.; Gargallo-Garriga, A.; Rattanapichai, W.; Oravec, M.; Holub, P.; Veselá, B.; Sardans, J.; Peñuelas, J.; Urban, O. Distinct Morphological, Physiological, and Biochemical Responses to Light Quality in Barley Leaves and Roots. Front. Plant Sci. 2019, 10, 1026. [Google Scholar] [CrossRef] [Green Version]
- Parida, A.K.; Panda, A.; Rangani, J. Metabolomics-Guided Elucidation of Abiotic Stress Tolerance Mechanisms in Plants. Plant Metab. Regul. Under Env. Stress 2018, 89–131. [Google Scholar] [CrossRef]
- Hare, P.; Cress, W.; van Staden, J. Proline synthesis and degradation: A model system for elucidating stress-related signal transduction. J. Exp. Bot. 1999, 50, 413–434. [Google Scholar] [CrossRef]
- Zinta, G.; AbcElgawad, H.; Peshev, D.; Weedon, J.T.; van den Ende, W.; Nijs, I.; Janssens, I.A.; Beemster, G.T.S.; Asard, H. Dynamics of metabolic responses to periods of combined heat and drought in Arabidopsis thaliana under ambient and elevated atmospherioc CO2. J. Exp. Bot. 2018, 69, 2159–2170. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef] [Green Version]
- Mundim, F.M.; Pringle, E.G. Whole-Plant Metabolic Allocation Under Water Stress. Front. Plant Sci. 2018, 9, 852. [Google Scholar] [CrossRef]
- Miura, K.; Tada, Y. Regulation of water, salinity, and cold stress responses by salicylic acid. Front. Plant Sci. 2014, 5, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Shan, C.; Liang, Z. Jasmonic acid regulates ascorbate and glutathione metabolism in Agropyron cristatum leaves under water stress. Plant Sci. 2010, 178, 130–139. [Google Scholar] [CrossRef]
- Mahouachi, J.; Arbona, V.; Gómez-Cadenas, A. Hormonal changes in papaya seedlings subjected to prograssive water stress in this halophyte. Plant Growth Regul. 2007, 53, 43–51. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Intergovernmental Panel on Climate Change (IPCC). Fourth Assessment Report. In Climate Change 2007: Synthesis Report; World Meteorological Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Nagarajan, S.; Jagadish, S.V.K.; Prasad, A.H.; Thomar, A.; Anand, A.; Pal, M.; Agarwal, P. Local climate affects growth, yield and grain quality of aromatic and non-aromatic rice in northwestern India. Agric. Ecosyst. Environ. 2010, 138, 274–281. [Google Scholar] [CrossRef]
- Scafaro, A.P.; Haynes, P.A.; Atwell, B.J. Physiological and molecular changes in Oryza meridionalis Ng., a heat-tolerant species of wild rice. J. Exp. Bot. 2010, 61, 191–202. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Li, J.; Zhang, X.; Wei, H.; Cui, L. Effects of heat acclimation pretreatment on changes of membrane lipid peroxidation, antioxidant metabolites, and ultrastructure of chloroplasts in two cool-season turfgrass species under heat stress. Environ. Exp. Bot. 2006, 56, 274–285. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox Regulation in Photosynthetic Organisms: Signaling, Acclimation, and Practical Implications. Antioxid. Redox Signal. 2009, 11, 861–905. [Google Scholar] [CrossRef]
- Suzuki, N.; Mittler, R. Reactive oxygen species and temperature stresses: A delicate balance between signaling and destruction. Physiol. Plant. 2006, 126, 45–51. [Google Scholar] [CrossRef]
- Potters, G.; Pasternak, T.P.; Guisez, Y.; Palme, K.J.; Jansen, M.A.K. Stress-induced mosphogenic responses: Growing out of trouble? Trends Plant Sci. 2007, 12, 98–105. [Google Scholar] [CrossRef]
- Wedow, J.M.; Yendrek, C.R.; Mello, T.R.; Creste, S.; Martinez, C.A.; Ainsworth, E.A. Metabolite, and transcript profiling of Guinea grass (Panicum maximum Jacq) response to elevated [CO2] and temperature. Metabolomics 2019, 15, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, X.; Xu, W.; Zhang, J.; Guo, R.; Zhao, M.; Hu, L.; Wang, H.; Dong, H.; Li, Y. Physiological characteristics and metabolomics of transgenic wheat containing the maize C4 phosphoenolpyruvate carboxylase (PEPC) gene under high temperature stress. Protoplasma 2016, 254, 1017–1030. [Google Scholar] [CrossRef] [PubMed]
- Carrow, R.N. Summer Decline of Bentgrass Greens; Golf Course Manager: Louisville, KY, USA, 1996; pp. 51–56. [Google Scholar]
- Huang, B.; Gao, H. Growth and Carbohydrate Metabolism of Creeping Bentgrass Cultivars in Response to Increasing Temperatures. Crop. Sci. 2000, 40, 1115–1120. [Google Scholar] [CrossRef]
- Youngner, V.B.; Nudge, F.J. Soil Temperature, Air Temperature, and Defoliation Effects on Growth and Nonstructural Carbohydrates of Kentucky Bluegrass1. Agron. J. 1907, 68, 257–260. [Google Scholar] [CrossRef]
- Song, S.Q.; Lei, Y.B.; Tian, X.R. Proline Metabolism and Cross-Tolerance to Salinity and Heat Stress in Germinating Wheat Seeds. Russ. J. Plant Physiol. 2005, 52, 793–800. [Google Scholar] [CrossRef]
- Yue, Y.; Jiang, H.; Du, J.; Shi, L.; Bin, Q.; Yang, X.; Wang, L. Variations in physiological response and expression profiles of proline metabolism-related genes and heat shock transcription factor genes in petunia subjected to heat stress. Sci. Hortic. 2019, 258, 108811. [Google Scholar] [CrossRef]
- Kishor, P.; Hong, Z.; Miao, G.H.; Hu, C.; Verma, D. Overexpression of [delta]-Pyrroline-5-Carboxylate Synthetase Increases Proline Production and Confers Osmotolerance in Transgenic Plants. Plant Physiol. 1995, 108, 1387–1394. [Google Scholar] [CrossRef] [Green Version]
- Solomon, A.; Beer, S.; Waisel, Y.; Jones, G.P.; Paleg, L.G. Effects of NaCl on the carboxylating activity of rubisco from Tamarix jordanis in the presence and absence of proline-related compatible solutes. Physiol. Plant. 1994, 90, 198–204. [Google Scholar] [CrossRef]
- Prasad, K.; Saradhi, P.P. Effect of zinc on free radicals and proline in Brassica and Cajanus. Phytochemisty 1995, 39, 45–47. [Google Scholar] [CrossRef]
- Kumar, D.; Chattopadhyay, S. Glutathione modulates the expression of heat shock proteins via the transcription factors BZIP10 and MYB21 in Arabidopsis. J. Exp. Bot. 2018, 69, 3729–3743. [Google Scholar] [CrossRef] [Green Version]
- Kocsy, G.; Szalai, G.; Galiba, G. Induction of Glutathione Synthesis and Glutathione Reductase Activity by Abiotic Stresses in Maize and Wheat. Sci. World J. 2002, 2, 1699–1705. [Google Scholar] [CrossRef] [Green Version]
- Locy, R.D.; Wu, S.-J.; Bisnette, J.; Barger, T.W.; McNabb, D.; Zik, M.; Fromm, H.; Singh, N.K.; Cherry, J.H. The Regulation of GABA Accumulation by Heat Stress in Arabidopsis. In Plant Tolerance to Abiotic Stresses in Agriculture: Role of Genetic Engineering; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2000; pp. 39–52. [Google Scholar]
- Rao, S.R.; Ravishankar, C. Enhanced catharanthine and vidoline production in suspension cultures of Catheranthus roseus by ultraviolet-B light. J. Mol. Signal. 2008, 3, 9–14. [Google Scholar]
- Yu, K.-W.; Murthy, H.N.; Hahn, E.-J.; Paek, K.Y. Ginsenoside production by hairy root cultures of Panax ginseng: Influence of temperature and light quality. Biochem. Eng. J. 2005, 23, 53–56. [Google Scholar] [CrossRef]
- Chan, L.K.; Koay, S.S.; Boey, P.L.; Bhatt, A. Effects of abiotic stress on biomass and anthocyanin production in cell cultures of Melastoma malabathricum. Boil. Res. 2010, 43, 127–135. [Google Scholar] [CrossRef] [Green Version]
- Zobayed, S.M.A.; Afreen, F.; Kozai, T. Temperature stress can alter the photosynthetic efficiency and secondary metabolite concentrations in St. John’s wort. Plant Physiol. Biochem. 2005, 43, 977–984. [Google Scholar] [CrossRef]
- Singsaas, E.L. Terpenes and thermotolerance of photosynthesis. New Phytol. 2000, 146, 1–4. [Google Scholar]
- Lichtenthaler, H.K.; Schwender, J.; Disch, A.; Rohmer, M. Biosynthesis of isoprenoids in higher plant chloroplasts proceeds via a mevalonate-independent pathway. FEBS Lett. 1997, 400, 271–274. [Google Scholar] [CrossRef] [Green Version]
- Henry, L.K.; Gutensohn, M.; Thomas, S.T.; Noel, J.P.; Duradeva, N. Orthologs of archael isopentenyl phosphate kinase regulate terpenoid production in plants. Proc. Nat. Acad. Sci. USA 2015, 112, 10050–10055. [Google Scholar]
- Sairam, R.; Tyagi, A. Physiology and molecular biology of salinity stress tolerance in plants. Curr. Sci. 2014, 86, 407–421. [Google Scholar]
- Peters, G.P.; Andrew, R.M.; Canadell, J.G.; Friedlingstein, P.; Jackson, R.B.; Korsbakken, J.I.; Le Quéré, C.; Peregon, A. Carbon dioxide emissions continue to grow amidst slowly emerging climate policies. Nat. Clim. Change 2019, 10, 3–6. [Google Scholar] [CrossRef]
- Duval, B.D.; Blankinship, J.C.; Dijkstra, P.; Hungate, B.A. Retracted Asticle: CO2 effects on plant nutrient concentration depend on plant functional group and available nitrogen: A meta-analysis. Plant Ecol. 2011, 213, 505–521. [Google Scholar] [CrossRef]
- Jin, J.; Tang, C.; Sale, P. The impact of elevated carbon dioxide on the phosphorus nutrition of plants: A review. Ann. Bot. 2015, 116, 987–999. [Google Scholar] [CrossRef] [Green Version]
- Hatfield, J.L.; Dold, C. Water-Use Efficiency: Advances and Challenges in a Changing Climate. Front. Plant Sci. 2019, 10, 103. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Jal-Ahammed, G.; Li, Z.X.; Wei, J.P.; Shen, C.; Yan, P.; Zhang, P.P.; Han, W.Y. Stimulation in primary and secondary metabolism by elevated carbon dioxide alters green tea quality in Camelia sinensis L. Sci. Rep. 2016, 7, 7937. [Google Scholar]
- Wenzel, S.; Cox, P.; Eyring, V.; Friedlingstein, P. Projected land photosynthesis constrained by changes in the seasonal cycle of atmospheric CO2. Nature 2016, 538, 499–501. [Google Scholar] [CrossRef]
- Penuelas, J.; Estiarte, M. Can elevated CO(2) affect secondary metabolism and ecosystem function? Trends Ecol. Evol. 1998, 13, 20–24. [Google Scholar] [CrossRef]
- Penuelas, J.; Estiarte, M.; Kimball, B. Flavonoid Responses in Wheat Grown at Elevated CO2: Green Versus Senescent Leaves. Photosynthetica 2000, 37, 615–619. [Google Scholar] [CrossRef]
- Peñuelas, J.; Fernández-Martínez, M.; Vallicrosa, H.; Maspons, J.; Zuccarini, P.; Carnicer, J.; Sanders, T.G.M.; Krüger, I.; Obersteiner, M.; Janssens, I.A.; et al. Increasing atmospheric CO2 concentrations correlate with declining nutritional status of European forests. Commun. Boil. 2020, 3, 1–11. [Google Scholar] [CrossRef]
- Peñuelas, J.; Janssens, I.A.; Ciais, P.; Obersteiner, M.; Sardans, J. Anthropogenic global shifts in biospheric N and P concentrations and ratios and their impacts on biodiversity, ecosystem productivity, food security, and human Health. Global Change Biol. 2020. [Google Scholar] [CrossRef] [Green Version]
- Lindroth, R.L.; Kinney, K.K.; Platz, C.L. Responses of deciduous trees to elevated atmospheric CO2, productivity, phytochemistry and insect performance. Ecology 1993, 4, 763–777. [Google Scholar]
- Penuelas, J.; Estiarte, M.; Llusià, J. Carbon-based Secondary Compounds at Elevated CO2. Photosynthetica 1997, 33, 313–319. [Google Scholar] [CrossRef]
- Penuelas, J.; Llusià, J. Effects of Carbon Dioxide, Water Supply, and Seasonality on Terpene Content and Emission by Rosmarinus officinalis. J. Chem. Ecol. 1997, 23, 979–993. [Google Scholar] [CrossRef] [Green Version]
- Penuelas, J.; Estiarte, M. Trends in plant carbon concentration and plant demand for N throughout this century. Oecologia 1996, 109, 69. [Google Scholar] [CrossRef] [PubMed]
- Holopainen, J.K.; Virjamo, V.; Ghimire, R.P.; Blande, J.D.; Julkunen-Tiitto, R.; Kivimäenpää, M. Climate Change Effects on Secondary Compounds of Forest Trees in the Northern Hemisphere. Front. Plant Sci. 2018, 9, 1445. [Google Scholar] [CrossRef] [Green Version]
- Koricheva, J.; Larsson, S.; Haukioja, E.; Keinänen, M. Regulation of Woody Plant Secondary Metabolism by Resource Availability: Hypothesis Testing by Means of Meta-Analysis. Oikos 1998, 83, 212. [Google Scholar] [CrossRef]
- Sobuj, N.; Virjamo, V.; Zhang, Y.; Nybakken, L.; Julkunen-Tiitto, R. Impacts of elevated temperature and CO2 concentration on growth and phenolics in the sexually dimorphic Populus tremula (L.). Environ. Exp. Bot. 2018, 146, 34–44. [Google Scholar] [CrossRef]
- Vanzo, E.; Jud, W.; Li, Z.; Albert, A.; Domagalska, M.A.; Ghirardo, A.; Niederbacher, B.; Frenzel, J.; Beemster, G.T.; Asard, H.; et al. Facing the Future: Effects of Short-Term Climate Extremes on Isoprene-Emitting and Nonemitting Poplar1. Plant Physiol. 2015, 169, 560–575. [Google Scholar] [CrossRef]
- Nissinen, K.; Nybakken, L.; Virjamo, V.; Julkunen-Tiitto, R. Slow-growing Salix repens (Salicaceae) benefits from changing climate. Environ. Exp. Bot. 2016, 128, 59–68. [Google Scholar] [CrossRef]
- McKiernan, A.B.; O’Reilly-Wapstra, J.; Price, C.; Davies, N.; Potts, B.; Hovenden, M.J. Stability of Plant Defensive Traits Among Populations in Two Eucalyptus Species Under Elevated Carbon Dioxide. J. Chem. Ecol. 2012, 38, 204–212. [Google Scholar] [CrossRef]
- Randriamanana, T.R.; Nissinen, K.; Ovaskainen, A.; Lavola, A.; Peltola, H.; Albrectsen, B.; Julkunen-Tiitto, R. Does fungal endophyte inoculation affect the responses of aspen seedlings to carbon dioxide enrichment? Fungal Ecol. 2018, 33, 24–31. [Google Scholar] [CrossRef]
- Llusià, J.; Peñuelas, J. Changes in terpene content and emission in potted Mediterranean woody plants under severe drought. Can. J. Bot. 1998, 8, 1366–1373. [Google Scholar]
- Peñuelas, J.; Llusià, J. BVOCs: Plant defense against climate warming? Trends Plant Sci. 2003, 3, 105–109. [Google Scholar]
- Blanch, J.S.; Penuelas, J.; Sardans, J.; Llusià, J. Drought, warming and soil fertilization effects on leaf volatile terpene concentrations in Pinus halepensis and Quercus ilex. Acta Physiol. Plant. 2008, 31, 207–218. [Google Scholar] [CrossRef]
- Bustos-Segura, C.; Dillon, S.; Keszei, A.; Foley, W.J.; Kulheim, C. Intraspecific diversity of terpenes of Eucalyptus camaldulensis (Myrtaceae) at a continental scale. Aust. J. Bot. 2017, 65, 257. [Google Scholar] [CrossRef]
- Templer, P.H.; Pinder, R.; Goodale, C.L. Effects of nitrogen deposition on greenhouse-gas fluxes for forests and grasslands of North America. Front. Ecol. Environ. 2012, 10, 547–553. [Google Scholar] [CrossRef]
- Carter, T.S.; Clark, C.M.; Fenn, M.E.; Jovan, S.; Perakis, S.S.; Riddell, J.; Schaberg, P.G.; Greaver, T.L.; Hastings, M.G. Mechanisms of nitrogen deposition effects on temperate forest lichens and trees. Ecosphere 2017, 8, e01717. [Google Scholar] [CrossRef]
- Schmitz, A.; Sanders, T.G.M.; Bolte, A.; Bussotti, F.; Dirnböck, T.; Johnson, J.; Peñuelas, J.; Pollastrini, M.; Prescher, A.-K.; Sardans, J.; et al. Responses of forest ecosystems in Europe to decreasing nitrogen deposition. Environ. Pollut. 2018, 244, 980–994. [Google Scholar] [CrossRef]
- Lassaletta, L.; Billen, G.; Grizzetti, B.; Anglade, J.; Garnier, J. 50-year trends in nitrogen use efficiency of world cropping systems: The relationship between yield and nitrogen input to cropland. Environ. Res. Lett. 2014, 9, 105011. [Google Scholar] [CrossRef]
- Bodirsky, B.L.; Müller, C. Robust relationship between yields and nitrogen inputs indicates three ways to reduce nitrogen pollution. Environ. Res. Lett. 2014, 9, 111005. [Google Scholar] [CrossRef] [Green Version]
- Larsen, S.U.; Jorgensen, H.; Bukh, C.; Schjoerring, J.K. Green biorefining: Effect of nitrogen fertilization on protein yield, protein extractability and amino acid composition of tall fescue biomass. Ind. Crop. Prod. 2019, 130, 642–652. [Google Scholar] [CrossRef]
- Huhn, G.; Schulz, H. Contents of free amino acids in Scots pine needles from field sites with different levels of nitrogen deposition. New Phytol. 1996, 134, 95–101. [Google Scholar] [CrossRef]
- Calanni, J.; Berg, E.; Wood, M.; Mangis, D.; Boyce, R.; Weathers, W.; Sievering, H. Atmospheric nitrogen deposition at a conifer forest: Response of free amino acids in Engelmann spruce needles. Environ. Pollut. 1999, 105, 79–89. [Google Scholar] [CrossRef]
- Limpens, J.; Berendse, F. Growth reduction of Sphagnum magellanicum subjected to high nitrogen deposition: The role of amino acid nitrogen concentration. Oecologia 2003, 135, 339–345. [Google Scholar] [CrossRef]
- Gent, M.P.N. Effect of Genotype, Fertilization, and Season on Free Amino Acids in Leaves of Salad Greens Grown in High Tunnels. J. Plant Nutr. 2005, 28, 1103–1116. [Google Scholar] [CrossRef]
- Ćustić, M.H.; Horvatić, M.; Pecina, M. Nitrogen Fertilization Influences Protein Nutritional Quality in Red Head Chicory. J. Plant Nutr. 2009, 32, 598–609. [Google Scholar] [CrossRef]
- Kanmegne, G.; Mbouobda, H.D.; Fotso-Mbakop, C.N.; Omokolo, D.N. The influence of stock plant fertilization on tissue concentrations of nitrogen, carbohydrates, and amino acids and on the rooting of leaf stem cuttings of Cola anomala K. Schum (Malvaceae). New For. 2017, 48, 17–31. [Google Scholar]
- Xu, Y.; Xiao, H. Free amino acid concentrations and nitrogen isotope signatures in Pinus massaniana (Lamb.) needles of different ages for indicating atmospheric nitrogen deposition. Env. Pollut. 2017, 221, 180–190. [Google Scholar]
- Nokerbekova, N.; Suleimenov, Y.T.; Zhapayev, R. Influence of Fertilizing with Nitrogen Fertilizer on the Content of Amino Acids in Sweet Sorghum Grain. Agric. Food Sci. Res. 2018, 5, 64–67. [Google Scholar] [CrossRef]
- Wen, G.; Cambouris, A.N.; Ziadi, N.; Bertrand, A.; Khelifi, M. Nitrogen Fertilization Effects on the Composition of Foliar Amino Acids of Russet Burbank Potato. Am. J. Potato Res. 2019, 96, 541–551. [Google Scholar] [CrossRef]
- Olsen, K.M.; Slimestad, R.; Lea, U.S.; Brede, C.; Løvdal, T.; Ruoff, P.; Verheul, M.; Lillo, C. Temperature and nitrogen effects on regulators and products of the flavonoid pathway: Experimental and kinetic model studies. Plant Cell Environ. 2009, 32, 286–299. [Google Scholar] [CrossRef]
- Allwood, J.W.; Chandra, S.; Xu, Y.; Dunn, W.B.; Correa, E.; Hopkins, L.; Goodacre, R.; Tobin, A.K.; Bowsher, C. Profiling of spatial metabolite distributions in wheat leaves under normal and nitrate limiting conditions. Phytochemisty 2015, 115, 99–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prinsi, B.; Espen, L. Mineral nitrogen sources differently affect root glutamine synthetase isoforms and amino acid balance among organs in maize. BMC Plant Boil. 2015, 15, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pietilä, M.; Lähdesmäki, P.; Pietiläinen, P.; Ferm, A.; Hytönen, J.; Pätilä, A. High nitrogen deposition causes changes in amino acid concentrations and protein spectra in needles of the scots pine (Pinus sylvestris). Environ. Pollut. 1991, 72, 103–115. [Google Scholar] [CrossRef]
- Ann-Brittedfast, T.N.; Ericsson, A.; Nordén, L.-G. Accumulation of amino acids in some boreal forest plants in response to increased nitrogen availability. New Phytol. 1994, 126, 137–143. [Google Scholar] [CrossRef]
- Cánovas, F.M.; Ávila, C.; Cantón, F.J.R.; Cañas, R.A.; De La Torre, F. Ammonium assimilation and amino acid metabolism in conifers. J. Exp. Bot. 2007, 58, 2307–2318. [Google Scholar] [CrossRef] [Green Version]
- Nordin, A.; Näsholm, T. Nitrogen storage forms in nine boreal understory plant species. Oecologia 1997, 110, 487–492. [Google Scholar] [CrossRef]
- Britto, D.T.; Siddiqi, M.Y.; Glass, A.D.M.; Kronzucker, H.J. Futile transmembrane NH4+ cycling: A cellular hypothesis to explain ammonium toxicity in plants. Proc. Natl. Acad. Sci. USA 2001, 98, 4255–4258. [Google Scholar] [CrossRef] [Green Version]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sardans, J.; Gargallo-Garriga, A.; Urban, O.; Klem, K.; Walker, T.W.N.; Holub, P.; Janssens, I.A.; Peñuelas, J. Ecometabolomics for a Better Understanding of Plant Responses and Acclimation to Abiotic Factors Linked to Global Change. Metabolites 2020, 10, 239. https://doi.org/10.3390/metabo10060239
Sardans J, Gargallo-Garriga A, Urban O, Klem K, Walker TWN, Holub P, Janssens IA, Peñuelas J. Ecometabolomics for a Better Understanding of Plant Responses and Acclimation to Abiotic Factors Linked to Global Change. Metabolites. 2020; 10(6):239. https://doi.org/10.3390/metabo10060239
Chicago/Turabian StyleSardans, Jordi, Albert Gargallo-Garriga, Otmar Urban, Karel Klem, Tom W.N. Walker, Petr Holub, Ivan A. Janssens, and Josep Peñuelas. 2020. "Ecometabolomics for a Better Understanding of Plant Responses and Acclimation to Abiotic Factors Linked to Global Change" Metabolites 10, no. 6: 239. https://doi.org/10.3390/metabo10060239
APA StyleSardans, J., Gargallo-Garriga, A., Urban, O., Klem, K., Walker, T. W. N., Holub, P., Janssens, I. A., & Peñuelas, J. (2020). Ecometabolomics for a Better Understanding of Plant Responses and Acclimation to Abiotic Factors Linked to Global Change. Metabolites, 10(6), 239. https://doi.org/10.3390/metabo10060239