Free Saturated Oxo Fatty Acids (SOFAs) and Ricinoleic Acid in Milk Determined by a Liquid Chromatography-High-Resolution Mass Spectrometry (LC-HRMS) Method
Abstract
:1. Introduction
2. Results
2.1. LC-ESI-MS Data
2.2. Method Validation
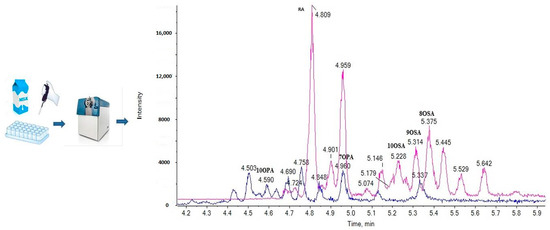
2.3. Liquid Chromatography
2.4. Suspect Analysis
2.5. Analysis of Samples
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Stock and Working Solutions
3.3. Instrumentation
3.4. Sample Preparation
3.5. Method Validation
3.6. Milk Samples
3.7. Statistics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jensen, R.G. The composition of bovine milk lipids: January 1995 to December 2000. J. Dairy Sci. 2002, 85, 295–350. [Google Scholar] [CrossRef]
- German, J.B.; Dillard, C.J. Composition, structure and absorption of milk lipids: A source of energy, fat-soluble nutrients and bioactive molecules. Crit. Rev. Food Sci. Nutr. 2006, 46, 57–92. [Google Scholar] [CrossRef] [PubMed]
- Mansson, L.H. Fatty acids in bovine milk fat. Food Nutr. Res. 2008, 52, 1821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De la Fuente, M.A.; Juarez, M. Fatty acids. In Handbook of Dairy Foods Analysis; Nollet, L.M.L., Toldrá, F., Eds.; CRC Press: Portland, OR, USA, 2009; pp. 211–228. [Google Scholar]
- Jenske, R.; Vetter, W.J. Enantioselective analysis of 2- and 3-hydroxy fatty acids in food samples. J. Agric. Food Chem. 2008, 56, 11578–11583. [Google Scholar] [CrossRef] [PubMed]
- Jenske, R.; Vetter, W. Concentrations of medium-chain 2- and 3-hydroxy fatty acids in foodstus. Food Chem. 2009, 114, 1122–1129. [Google Scholar] [CrossRef]
- Márquez-Ruiz, G.; Rodríguez-Pino, V.; de la Fuente, M.A. Determination of 10-hydroxystearic, 10-ketostearic, 8-hydroxypalmitic, and 8-ketopalmitic acids in milk fat by solid-phase extraction plus gas chromatography-mass spectrometry. J. Dairy Sci. 2011, 94, 4810–4819. [Google Scholar] [CrossRef] [Green Version]
- Kokotou, M.G.; Kokotos, A.C.; Gkikas, D.; Mountanea, O.G.; Mantzourani, C.; Almutairi, A.; Lei, X.; Ramanadham, S.; Politis, P.K.; Kokotos, G. Saturated hydroxy fatty acids exhibit a cell growth inhibitory activity and suppress the cytokine-induced β-cell apoptosis. J. Med. Chem. 2020, 63, 12666–12681. [Google Scholar] [CrossRef]
- Kokotou, M.G.; Mantzourani, C.; Bourboula, A.; Mountanea, O.G.; Kokotos, G. A liquid chromatography-high resolution mass spectrometry (LC-HRMS) method for the determination of free hydroxy fatty acids in cow and goat milk. Molecules 2020, 25, 3947. [Google Scholar] [CrossRef]
- Zhu, Q.-F.; An, N.; Feng, Y.-Q. In-Depth annotation strategy of saturated hydroxy fatty acids based on their chromatographic retention behaviors and MS fragmentation patterns. Anal. Chem. 2020, 21, 14528–14535. [Google Scholar] [CrossRef]
- Garton, G.A. The composition and bio-synthesis of milk lipids. J. Lipid Res. 1963, 4, 237–254. [Google Scholar] [CrossRef]
- Katz, I.; Keeney, M. Occurrence of ketostearic acids in the rumen. J. Dairy Sci. 1966, 49, 967–970. [Google Scholar] [CrossRef]
- Gómez-Cortés, P.; Juárez, M.; de la Fuente, M.A. Milk fatty acids and potential health benefits: An updated vision. Trends Food Sci. Technol. 2018, 81, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Amores, G.; Virto, M. Total and free fatty acids analysis in milk and dairy fat. Separations 2019, 6, 14. [Google Scholar] [CrossRef] [Green Version]
- Christie, W.W. Gas chromatography-mass spectrometry methods for structural analysis of fatty acids. Lipids 1998, 33, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Mannion, D.T.; Furey, A.; Kilcawley, K.N. Free fatty acids quantification in dairy products. Int. J. Dairy Technol. 2016, 69, 1–12. [Google Scholar] [CrossRef] [Green Version]
- La Nasa, J.; Degano, I.; Brandolini, L.; Modugno, F.; Bonaduce, I. A novel HPLC-ESI-Q-ToF approach for the determination of fatty acids and acylglycerols in food samples. Anal. Chim. Acta 2018, 1013, 98–109. [Google Scholar] [CrossRef]
- Kokotou, M.G.; Mantzourani, C.; Kokotos, G. Development of a liquid chromatography-high resolution mass spectrometry method for the determination of free fatty acids in milk. Molecules 2020, 25, 1548. [Google Scholar] [CrossRef] [Green Version]
- Dorsey, J.G.; Dill, K.A. The molecular mechanism of retention in reversed-phase liquid chromatography. Chem. Rev. 1989, 89, 331–346. [Google Scholar] [CrossRef]
- De Oliveira Maia Parente, M.; Susin, I.; Nolli, P.C.; Ferreira, M.E.; Gentil, S.R.; Polizel, M.D.; Pires, V.A.; Alves, S.P.; Bessa, R.J.B. Effects of supplementation with vegetable oils, including castor oil, on milk production of ewes and on growth of their lambs. J. Anim. Sci. 2018, 96, 354–363. [Google Scholar] [CrossRef] [Green Version]
- Gandra, J.R.; Nunes Gil, P.C.; Gandra, E.R.S.; del Vale, T.A.; Barletta, R.V.; Zanferari, F.; de Jesus, F.E.; Takiya, C.S.; Mingoti, R.D.; Almeida, G.F.; et al. Productive performance of simmental dairy cows supplemented with ricinoleic acid from castor oil. Arch. Zootec. 2014, 63, 575–585. [Google Scholar] [CrossRef] [Green Version]
- Hill, H.M.; Bakes, D.; Love, I. Manual chromatographic baseline integration: Is it needed, if so when should it be used? Bioanalysis 2014, 6, 1171–1174. [Google Scholar] [CrossRef] [PubMed]
- Hudson, J.A.; MacKenzie, C.A.M.; Joblin, K.N. Conversion of oleic acid to 10-hydroxystearic acid by two species of ruminal bacteria. Appl. Microbiol. Biotechnol. 1995, 44, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hudson, J.A.; Cai, Y.; Corner, R.J.; Morvan, B.; Joblin, K.N. Identification and enumeration of oleic and linoleic acid hydrating bacteria in the rumen of sheep and cows. J. Appl. Bacteriol. 2000, 88, 286–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkins, T.C.; AbuGhazaleh, A.A.; Freeman, S.; Thies, E.J. The production of 10-hydroxystearic and 10-ketostearic acids is an alternative route of oleic acid transformation by the ruminal microbiota in cattle. J. Nutr. 2006, 136, 926–931. [Google Scholar] [CrossRef] [Green Version]
- McKain, N.; Shingfield, K.J.; Wallace, R.J. Metabolism of conjugated linoleic acids and 18:1 fatty acids by ruminal bacteria: Products and mechanisms. Microbiology 2010, 156, 579–588. [Google Scholar] [CrossRef] [Green Version]
- Alves, S.P.; Araujo, C.M.; Queiroga, R.C.; Madruga, M.S.; Parente, M.O.M.; Medeiros, A.N.; Bessa, R.J.B. New insights on the metabolism of ricinoleic acid in ruminants. J. Dairy Sci. 2017, 100, 8018–8032. [Google Scholar] [CrossRef] [Green Version]
- Guidance for Industry. Q2B Validation of analytical procedures: Methodology; International Council for Harmonization (ICH): Geneva, Switzerland, 1996.




| Compound | Abbreviation | Structure | Theoretical Mass [M − H]− | Mass Error (ppm) | Retention Time (min) |
|---|---|---|---|---|---|
| 14-Oxopalmitic acid | 14OPA |  | 269.2122 | 0.74 | 4.47 |
| 10-Oxopalmitic acid | 10OPA |  | 269.2122 | 0.85 | 4.60 |
| 9-Oxopalmitic acid | 9OPA |  | 269.2122 | 0.80 | 4.66 |
| 8-Oxopalmitic acid | 8OPA |  | 269.2122 | 0.69 | 4.73 |
| 7-Oxopalmitic acid | 7OPA |  | 269.2122 | 0.75 | 4.81 |
| 6-Oxopalmitic acid | 6OPA |  | 269.2122 | 0.82 | 4.93 |
| 16-Oxostearic acid | 16OSA |  | 297.2435 | 1.01 | 5.11 |
| 12-Oxostearic acid | 12OSA |  | 297.2435 | 1.11 | 5.19 |
| 10-Oxostearic acid | 10OSA |  | 297.2435 | 0.92 | 5.28 |
| 9-Oxostearic acid | 9OSA |  | 297.2435 | 0.95 | 5.34 |
| 8-Oxostearic acid | 8OSA |  | 297.2435 | 1.09 | 5.41 |
| 7-Oxostearic acid | 7OSA |  | 297.2435 | 1.10 | 5.49 |
| 6-Oxostearic acid | 6OSA |  | 297.2435 | 1.03 | 5.60 |
| Analyte | Linearity (R2) | LOD (ng/mL) | LOQ (ng/mL) |
|---|---|---|---|
| 14OPA | 0.990 | 0.3 | 1.0 |
| 10OPA | 0.993 | 0.5 | 1.4 |
| 9OPA | 0.995 | 0.3 | 1.0 |
| 8OPA | 0.992 | 0.5 | 1.4 |
| 7OPA | 0.993 | 0.5 | 1.4 |
| 6OPA | 0.990 | 0.5 | 1.4 |
| 16OSA | 0.991 | 0.8 | 2.4 |
| 12OSA | 0.990 | 0.5 | 1.4 |
| 10OSA | 0.992 | 0.5 | 1.4 |
| 9OSA | 0.995 | 0.5 | 1.4 |
| 8OSA | 0.991 | 0.5 | 1.4 |
| 7OSA | 0.991 | 0.8 | 2.4 |
| 6OSA | 0.990 | 0.8 | 2.4 |
| RA | 0.996 | 0.6 | 2.0 |
| Analyte | Spike Level (ng/mL) | Recovery (%) | MF | RSDr (%) | RSDR (%) |
|---|---|---|---|---|---|
| 14OPA | 50 | 79.3 | 0.7 | 5.3 | 7.3 |
| 200 | 82.3 | 0.8 | 0.7 | 5.8 | |
| 500 | 81.7 | 0.9 | 5.9 | 6.3 | |
| 10OPA | 50 | 82.9 | 0.7 | 7.9 | 10.1 |
| 200 | 99.5 | 1.0 | 6.1 | 11.3 | |
| 500 | 83.1 | 0.9 | 1.6 | 11.5 | |
| 9OPA | 50 | 79.1 | 0.7 | 4.4 | 3.7 |
| 200 | 86.7 | 0.9 | 6.4 | 5.1 | |
| 500 | 84.1 | 0.7 | 0.9 | 2.3 | |
| 8OPA | 50 | 79.1 | 0.6 | 3.7 | 2.1 |
| 200 | 82.4 | 0.9 | 0.5 | 1.1 | |
| 500 | 84.7 | 0.8 | 2.7 | 0.8 | |
| 7OPA | 50 | 77.0 | 0.7 | 9.3 | 11.1 |
| 200 | 75.9 | 0.9 | 7.2 | 7.7 | |
| 500 | 87.7 | 0.7 | 5.0 | 10.3 | |
| 6OPA | 50 | 73.4 | 0.7 | 5.9 | 4.9 |
| 200 | 73.9 | 0.8 | 7.2 | 6.1 | |
| 500 | 82.4 | 0.7 | 1.1 | 6.5 | |
| 16OSA | 50 | 81.2 | 0.7 | 7.2 | 10.4 |
| 200 | 90.9 | 0.9 | 3.2 | 8.3 | |
| 500 | 112.0 | 1.0 | 0.1 | 6.9 | |
| 12OSA | 50 | 83.3 | 0.7 | 4.3 | 1.9 |
| 200 | 84.2 | 0.8 | 2.1 | 4.9 | |
| 500 | 102.9 | 1.0 | 1.7 | 2.0 | |
| 10OSA | 50 | 76.3 | 0.8 | 7.3 | 11.1 |
| 200 | 81.9 | 0.9 | 7.7 | 3.9 | |
| 500 | 87.5 | 0.8 | 5.3 | 7.4 | |
| 9OSA | 50 | 78.4 | 0.7 | 2.5 | 10.1 |
| 200 | 74.5 | 0.7 | 6.8 | 10.3 | |
| 500 | 96.2 | 0.9 | 1.2 | 11.2 | |
| 8OSA | 50 | 74.2 | 0.7 | 5.8 | 4.4 |
| 200 | 83.4 | 0.7 | 2.1 | 5.5 | |
| 500 | 94.9 | 1.1 | 2.2 | 1.0 | |
| 7OSA | 50 | 81.6 | 0.7 | 3.5 | 10.3 |
| 200 | 81.5 | 0.8 | 6.8 | 10.8 | |
| 500 | 91.3 | 1.1 | 3.2 | 11.1 | |
| 6OSA | 50 | 84.0 | 0.7 | 3.3 | 7.2 |
| 200 | 95.4 | 0.9 | 4.8 | 6.4 | |
| 500 | 96.1 | 1.1 | 1.3 | 8.2 | |
| RA | 50 | 90.1 | 1.0 | 6.6 | 1.5 |
| 200 | 89.7 | 0.8 | 3.2 | 4.1 | |
| 500 | 99.2 | 0.9 | 4.1 | 3.0 |
| Cow Milk (n = 12), Triplicates | Goat Milk (n = 8), Triplicates | |||||||
|---|---|---|---|---|---|---|---|---|
| SOFAs and RA | Minimum Value (μg/mL) | Maximum Value (μg/mL) | Mean Value ± SD (μg/mL) | Level of Significance | Minimum Value (μg/mL) | Maximum Value (μg/mL) | Mean Value ± SD (μg/mL) | Level of Significance |
| 14OPA | 8.1 | 32.4 | 22.3 ± 4.0 | *** | 6.6 | 23.7 | 15.4 ± 3.1 | *** |
| 10OPA | 31.7 | 127.5 | 76.7 ± 7.2 | *** | <LOQ e | 84.6 | 41.9 ± 5.6 | ** |
| 9OPA | 9.0 | 55.4 | 23.3 ± 5.6 | *** | <LOQ e | 37.8 | 17.3 ± 4.1 | ** |
| 8OPA | 6.4 | 48.5 | 14.7 ± 3.1 | *** | <LOQ e | 18.5 | 9.4 ± 1.6 | *** |
| 7OPA | <LOQ a | 111.6 | 43.6 ± 5.2 | ** | <LOQ e | 20.3 | 15.0 ± 3.1 | ** |
| 6OPA | <LOQ b | 52.3 | 12.5 ± 4.1 | ** | 4.4 | 23.1 | 1.6 ± 2.9 | ** |
| 16OSA | <LOQ c | 30.3 | 6.5 ± 6.1 | ** | <LOQ | <LOQ | - | - |
| 12OSA | <LOQ d | 59.9 | 17.4 ± 5.1 | ** | <LOQ a | 18.0 | 6.8 ± 5.3 | * |
| 10OSA | <LOQ e | 118.3 | 47.6 ± 4.6 | *** | <LOQ a | 53.8 | 20.3 ± 5.1 | * |
| 9OSA | 26.9 | 176.8 | 89.0 ± 6.5 | *** | 46.6 | 85.3 | 66.9 ± 7.1 | *** |
| 8OSA | <LOQ b | 239.5 | 96.9 ± 7.2 | ** | <LOQ a | 114.1 | 45.6 ± 8.0 | * |
| 7OSA | <LOQ b | 120.6 | 66.8 ± 6.5 | ** | <LOQ a | 101.2 | 42.1 ± 7.2 | * |
| 6OSA | <LOQ | <LOQ | - | - | <LOQ | <LOQ | - | - |
| RA | 181.7 | 620.2 | 534.3 ± 6.0 | *** | 231.1 | 411.2 | 460.4 ± 8.1 | *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kokotou, M.G.; Batsika, C.S.; Mantzourani, C.; Kokotos, G. Free Saturated Oxo Fatty Acids (SOFAs) and Ricinoleic Acid in Milk Determined by a Liquid Chromatography-High-Resolution Mass Spectrometry (LC-HRMS) Method. Metabolites 2021, 11, 46. https://doi.org/10.3390/metabo11010046
Kokotou MG, Batsika CS, Mantzourani C, Kokotos G. Free Saturated Oxo Fatty Acids (SOFAs) and Ricinoleic Acid in Milk Determined by a Liquid Chromatography-High-Resolution Mass Spectrometry (LC-HRMS) Method. Metabolites. 2021; 11(1):46. https://doi.org/10.3390/metabo11010046
Chicago/Turabian StyleKokotou, Maroula G., Charikleia S. Batsika, Christiana Mantzourani, and George Kokotos. 2021. "Free Saturated Oxo Fatty Acids (SOFAs) and Ricinoleic Acid in Milk Determined by a Liquid Chromatography-High-Resolution Mass Spectrometry (LC-HRMS) Method" Metabolites 11, no. 1: 46. https://doi.org/10.3390/metabo11010046
APA StyleKokotou, M. G., Batsika, C. S., Mantzourani, C., & Kokotos, G. (2021). Free Saturated Oxo Fatty Acids (SOFAs) and Ricinoleic Acid in Milk Determined by a Liquid Chromatography-High-Resolution Mass Spectrometry (LC-HRMS) Method. Metabolites, 11(1), 46. https://doi.org/10.3390/metabo11010046