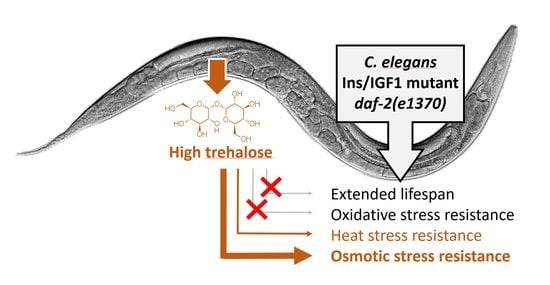
Elevated Trehalose Levels in C. elegans daf-2 Mutants Increase Stress Resistance, Not Lifespan
Abstract
:1. Introduction
2. Results
2.1. Expression Pattern of Trehalose Phosphate Synthases
2.2. Longevity of daf-2 Mutants Is Independent of tps Activity
2.3. tps-1/2 RNAi in daf-2 Mutants Reduces Trehalose to Wild-Type Levels
2.4. Maltose, Glucose, and Glycogen Do Not Compensate for Trehalose Reduction
2.5. Trehalose Is Required for Increased Osmotic and Heat Stress Resistance in daf-2 Mutants
3. Discussion
4. Materials and Methods
4.1. C. elegans Strains and Culture Conditions
4.2. RNAi Assay
4.3. Microscopy
4.4. Lifespan Assay
4.5. Stress Resistance Assays
4.5.1. Osmotic Stress Assay
4.5.2. Heat and Oxidative Stress Assays
4.6. Carbohydrate Determination in Worm Extracts
4.7. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kenyon, C.; Chang, J.; Gensch, E.; Rudner, A.; Tabtiang, R. A C. elegans mutant that lives twice as long as wild type. Nature 1993, 366, 461–464. [Google Scholar] [CrossRef]
- Henderson, S.T.; Johnson, T.E. daf-16 integrates developmental and environmental inputs to mediate aging in the nematode Caenorhabditis elegans. Curr. Biol. 2001, 11, 1975–1980. [Google Scholar] [CrossRef] [Green Version]
- Lin, K.; Hsin, H.; Libina, N.; Kenyon, C. Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-1 and germline signaling. Nat. Genet. 2001, 28, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.T.; McCarroll, S.A.; Bargmann, C.I.; Fraser, A.; Kamath, R.S.; Ahringer, J.; Li, H.; Kenyon, C. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature 2003, 424, 277–283. [Google Scholar] [CrossRef]
- McElwee, J.J.; Schuster, E.; Blanc, E.; Thomas, J.H.; Gems, D. Shared transcriptional signature in Caenorhabditis elegans dauer larvae and long-lived daf-2 mutants implicates detoxification system in longevity assurance. J. Biol. Chem. 2004, 279, 44533–44543. [Google Scholar] [CrossRef] [Green Version]
- Ogg, S.; Paradis, S.; Gottlieb, S.; Patterson, G.I.; Lee, L.; Tissenbaum, H.A.; Ruvkun, G. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature 1997, 389, 994–999. [Google Scholar] [CrossRef]
- Frazier, H.N.; Roth, M.B. Adaptive Sugar Provisioning Controls Survival of C. elegans Embryos in Adverse Environments. Curr. Biol. 2009, 19, 859–863. [Google Scholar] [CrossRef] [Green Version]
- Depuydt, G.; Xie, F.; Petyuk, V.A.; Smolders, A.; Brewer, H.M.; Camp, D.G.; Smith, R.D.; Braeckman, B.P. LC-MS proteomics analysis of the insulin/IGF-1-deficient Caenorhabditis elegans daf-2(e1370) mutant reveals extensive restructuring of intermediary metabolism. J. Proteome Res. 2014, 13. [Google Scholar] [CrossRef] [PubMed]
- Walther, D.M.; Kasturi, P.; Zheng, M.; Pinkert, S.; Vecchi, G.; Ciryam, P.; Morimoto, R.I.; Dobson, C.M.; Vendruscolo, M.; Mann, M.; et al. Widespread proteome remodeling and aggregation in aging C. elegans. Cell 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lithgow, G.J.; White, T.M.; Melov, S.; Johnson, T.E. Thermotolerance and extended life-span conferred by single-gene mutations and induced by thermal stress. Proc. Natl. Acad. Sci. USA 1995, 92, 7540–7544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamitina, S.T.; Strange, K. Transcriptional targets of DAF-16 insulin signaling pathway protect C. elegans from extreme hypertonic stress. AJP Cell Physiol. 2005, 288, C467–C474. [Google Scholar] [CrossRef]
- Scott, B.A. Regulation of Hypoxic Death in C. elegans by the Insulin/IGF Receptor Homolog DAF-2. Science 2002, 296, 2388–2391. [Google Scholar] [CrossRef]
- Murakami, S.; Johnson, T.E. A genetic pathway conferring life extension and resistance to UV stress in Caenorhabditis elegans. Genetics 1996, 143, 1207–1218. [Google Scholar] [CrossRef]
- Barsyte, D.; Lovejoy, D.; Lithgow, G. Longevity and heavy metal resistance in daf-2 and age-1 long-lived mutants of Caenorhabditis elegans. FASEB J. 2001, 15, 627–634. [Google Scholar] [CrossRef] [Green Version]
- Garsin, D.A. Long-Lived C. elegans daf-2 Mutants Are Resistant to Bacterial Pathogens. Science 2003, 300, 1921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanfleteren, J.R. Oxidative stress and ageing in Caenorhabditis elegans. Biochem. J. 1993, 292, 605–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balch, W.E.; Morimoto, R.I.; Dillin, A.; Kelly, J.W. Adapting Proteostasis for Disease Intervention. Science 2008, 319, 916–919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lithgow, G.J.; Walker, G.A. Stress resistance as a determinate of C. elegans lifespan. Mech. Ageing Dev. 2002, 123, 765–771. [Google Scholar] [CrossRef]
- Dues, D.J.; Andrews, E.K.; Senchuk, M.M.; Van Raamsdonk, J.M. Resistance to stress can be experimentally dissociated from longevity. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, S.K.; Bundy, J.G.; Leroi, A.M. Metabolic Youth in Middle Age: Predicting Aging in Caenorhabditis elegans Using Metabolomics. J. Proteome Res. 2015, 14, 4603–4609. [Google Scholar] [CrossRef] [Green Version]
- Fuchs, S.; Bundy, J.G.; Davies, S.K.; Viney, J.M.; Swire, J.S.; Leroi, A.M. A metabolic signature of long life in Caenorhabditis elegans. BMC Biol. 2010, 8, 14. [Google Scholar] [CrossRef] [Green Version]
- Honda, Y.; Tanaka, M.; Honda, S. Trehalose extends longevity in the nematode Caenorhabditis elegans. Aging Cell 2010, 9, 558–569. [Google Scholar] [CrossRef]
- Seo, Y.; Kingsley, S.; Walker, G.; Mondoux, M.A.; Tissenbaum, H.A. Metabolic shift from glycogen to trehalose promotes lifespan and healthspan in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2018, 115, E2791–E2800. [Google Scholar] [CrossRef] [Green Version]
- McElwee, J.; Bubb, K.; Thomas, J.H. Transcriptional outputs of the Caenorhabditis elegans forkhead protein DAF-16. Aging Cell 2003, 2, 111–121. [Google Scholar] [CrossRef] [Green Version]
- McElwee, J.J.; Schuster, E.; Blanc, E.; Thornton, J.; Gems, D. Diapause-associated metabolic traits reiterated in long-lived daf-2 mutants in the nematode Caenorhabditis elegans. Mech. Ageing Dev. 2006, 127, 458–472. [Google Scholar] [CrossRef]
- Elbein, A.D. New insights on trehalose: A multifunctional molecule. Glycobiology 2003, 13, 17R–27R. [Google Scholar] [CrossRef]
- Erkut, C.; Penkov, S.; Khesbak, H.; Vorkel, D.; Verbavatz, J.-M.; Fahmy, K.; Kurzchalia, T.V. Trehalose Renders the Dauer Larva of Caenorhabditis elegans Resistant to Extreme Desiccation. Curr. Biol. 2011, 21, 1331–1336. [Google Scholar] [CrossRef] [Green Version]
- De Virgilio, C.; Hottiger, T.; Dominiguez, J.; Boller, T.; Wiemken, A. The role of trehalose synthesis for the acquisition of thermotolerance in yeast: I. Genetic evidence that trehalose is a thermoprotectant. Eur. J. Biochem. 1994, 219, 179–186. [Google Scholar] [CrossRef]
- Watanabe, M.; Kikawada, T.; Minagawa, N.; Yukuhiro, F.; Okuda, T. Mechanism allowing an insect to survive complete dehydration and extreme temperatures. J. Exp. Biol. 2002, 205, 2799–2802. [Google Scholar]
- Jagdale, G.B.; Grewal, P.S.; Salminen, S.O. Both heat-shock and cold-shock influence trehalose metabolism in an entomopathogenic nematode. J. Parasitol. 2005, 91, 988–994. [Google Scholar] [CrossRef]
- Sakurai, M.; Furuki, T.; Akao, K.-I.; Tanaka, D.; Nakahara, Y.; Kikawada, T.; Watanabe, M.; Okuda, T. Vitrification is essential for anhydrobiosis in an African chironomid, Polypedilum vanderplanki. Proc. Natl. Acad. Sci. USA 2008, 105, 5093–5098. [Google Scholar] [CrossRef] [Green Version]
- Jain, N.K.; Roy, I. Effect of trehalose on protein structure. Protein Sci. 2008, 18. [Google Scholar] [CrossRef]
- Hottiger, T.; Virgilio, C.; Hall, M.N.; Boller, T.; Wiemken, A. The role of trehalose synthesis for the acquisition of thermotolerance in yeast. II. Physiological concentrations of trehalose increase the thermal stability of proteins in vitro. Eur. J. Biochem. 1994, 219, 187–193. [Google Scholar] [CrossRef]
- Depuydt, G.; Shanmugam, N.; Rasulova, M.; Dhondt, I.; Braeckman, B.P. Increased protein stability and decreased protein turnover in the Caenorhabditis elegans ins/igf-1 daf-2 mutant. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2016, 71, 1553–1559. [Google Scholar] [CrossRef] [Green Version]
- Hutter, H.; Suh, J. GExplore 1.4: An expanded web interface for queries on Caenorhabditis elegans protein and gene function. Worm 2016. [Google Scholar] [CrossRef] [Green Version]
- Penkov, S.; Kaptan, D.; Erkut, C.; Sarov, M.; Mende, F.; Kurzchalia, T.V. Integration of carbohydrate metabolism and redox state controls dauer larva formation in Caenorhabditis elegans. Nat. Commun. 2015, 6, 8060. [Google Scholar] [CrossRef] [Green Version]
- Hibshman, J.D.; Doan, A.E.; Moore, B.T.; Kaplan, R.E.; Hung, A.; Webster, A.K.; Bhatt, D.P.; Chitrakar, R.; Hirschey, M.D.; Baugh, L.R. daf-16/FoxO promotes gluconeogenesis and trehalose synthesis during starvation to support survival. Elife 2017. [Google Scholar] [CrossRef]
- Chiang, W.C.; Tishkoff, D.X.; Yang, B.; Wilson-Grady, J.; Yu, X.; Mazer, T.; Eckersdorff, M.; Gygi, S.P.; Lombard, D.B.; Hsu, A.L. C. elegans SIRT6/7 Homolog SIR-2.4 Promotes DAF-16 Relocalization and Function during Stress. PLoS Genet. 2012. [Google Scholar] [CrossRef]
- Houthoofd, K.; Braeckman, B.P.; Johnson, T.E.; Vanfleteren, J.R. Life extension via dietary restriction is independent of the Ins/IGF-1 signalling pathway in Caenorhabditis elegans. Exp. Gerontol. 2003, 38. [Google Scholar] [CrossRef]
- Cooper, A.F.; Van Gundy, S.D. Metabolism of Glycogen and Neutral Lipids by Aphelenchus avenae and Caenorhabditis sp. in Aerobic, Microaerobic and Anaerobic Environments. J. Nematol. 1970, 2, 305–315. [Google Scholar]
- Föll, R.L.; Pleyers, A.; Lewandovski, G.J.; Wermter, C.; Hegemann, V.; Paul, R.J. Anaerobiosis in the nematode Caenorhabditis elegans. Comp. Biochem. Physiol. Part. B Biochem. Mol. Biol. 1999, 124, 269–280. [Google Scholar] [CrossRef]
- Braeckman, B.P.; Dhondt, I. Lifespan extension in Caenorhabditis elegans insulin/IGF-1 signalling mutants is supported by non-vertebrate physiological?traits. Nematology 2017, 19, 499–508. [Google Scholar] [CrossRef] [Green Version]
- Gusarov, I.; Pani, B.; Gautier, L.; Smolentseva, O.; Eremina, S.; Shamovsky, I.; Katkova-Zhukotskaya, O.; Mironov, A.; Nudler, E. Glycogen controls Caenorhabditis elegans lifespan and resistance to oxidative stress. Nat. Commun. 2017, 8, 15868. [Google Scholar] [CrossRef]
- Benedetto, A.; Bambade, T.; Au, C.; Tullet, J.M.A.; Monkhouse, J.; Dang, H.; Cetnar, K.; Chan, B.; Cabreiro, F.; Gems, D. New label-free automated survival assays reveal unexpected stress resistance patterns during C. elegans aging. Aging Cell 2019, 18, e12998. [Google Scholar] [CrossRef] [Green Version]
- Schulenburg, H.; Félix, M.A. The natural biotic environment of Caenorhabditis elegans. Genetics 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burnell, A.M.; Houthoofd, K.; O’Hanlon, K.; Vanfleteren, J.R. Alternate metabolism during the dauer stage of the nematode Caenorhabditis elegans. Exp. Gerontol. 2005, 40, 850–856. [Google Scholar] [CrossRef] [Green Version]
- Galimov, E.R.; Gems, D. Shorter life and reduced fecundity can increase colony fitness in virtual Caenorhabditis elegans. Aging Cell 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, K.I.; Pincus, Z.; Slack, F.J. Longevity and stress in Caenorhabditis elegans. Aging 2011, 3, 733–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hazell, B.W.; Nevalainen, H.; Attfield, P.V. Evidence that the Saccharomyces cerevisiae CIF1 (GGS1/TPS1) gene modulates heat shock response positively. FEBS Lett. 1995. [Google Scholar] [CrossRef] [Green Version]
- Rizki, G.; Iwata, T.N.; Li, J.; Riedel, C.G.; Picard, C.L.; Jan, M.; Murphy, C.T.; Lee, S.S. The evolutionarily conserved longevity determinants HCF-1 and SIR-2.1/SIRT1 collaborate to regulate DAF-16/FOXO. PLoS Genet. 2011. [Google Scholar] [CrossRef] [Green Version]
- Seo, M.; Seo, K.; Hwang, W.; Koo, H.J.; Hahm, J.H.; Yang, J.S.; Han, S.K.; Hwang, D.; Kim, S.; Jang, S.K.; et al. RNA helicase HEL-1 promotes longevity by specifically activating DAF-16/FOXO transcription factor signaling in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonoda, S.; Ohta, A.; Maruo, A.; Ujisawa, T.; Kuhara, A. Sperm Affects Head Sensory Neuron in Temperature Tolerance of Caenorhabditis elegans. Cell Rep. 2016. [Google Scholar] [CrossRef] [Green Version]
- Senchuk, M.M.; Dues, D.J.; Schaar, C.E.; Johnson, B.K.; Madaj, Z.B.; Bowman, M.J.; Winn, M.E.; Van Raamsdonk, J.M. Activation of DAF-16/FOXO by reactive oxygen species contributes to longevity in long-lived mitochondrial mutants in Caenorhabditis elegans. PLoS Genet. 2018. [Google Scholar] [CrossRef]
- Feng, Y. Study of Glucose Transporters in C. elegans. Ph.D. Thesis, University of Bath, Bath, UK, 2010. [Google Scholar]
- Kitaoka, S.; Morielli, A.D.; Zhao, F.Q. FGT-1-mediated glucose uptake is defective in insulin/IGF-like signaling mutants in Caenorhabditis elegans. FEBS Open Bio 2016. [Google Scholar] [CrossRef] [Green Version]
- Kitaoka, S.; Morielli, A.D.; Zhao, F.Q. FGT-1 Is a Mammalian GLUT2-Like Facilitative Glucose Transporter in Caenorhabditis elegans Whose Malfunction Induces Fat Accumulation in Intestinal Cells. PLoS ONE 2013. [Google Scholar] [CrossRef] [PubMed]
- Somerville, A.C. Trehalose Catabolism in the Nematode Caenorhabditis elegans. Ph.D. Thesis, Australian National University, Canberra, Australia, 1999. [Google Scholar]
- Kimble, J.; Sharrock, W.J. Tissue-specific synthesis of yolk proteins in Caenorhabditis elegans. Dev. Biol. 1983, 96, 189–196. [Google Scholar] [CrossRef]
- Rompay, L.; Van Borghgraef, C.; Beets, I.; Caers, J.; Temmerman, L. New genetic regulators question relevance of abundant yolk protein production in C. elegans. Sci. Rep. 2015. [Google Scholar] [CrossRef] [Green Version]
- Erkut, C.; Gade, V.R.; Laxman, S.; Kurzchalia, T.V. The glyoxylate shunt is essential for desiccation tolerance in C. elegans and budding yeast. Elife 2016, 5, 1–24. [Google Scholar] [CrossRef]
- Kamath, R.S.; Martinez-Campos, M.; Zipperlen, P.; Fraser, A.G.; Ahringer, J. Effectiveness of specific RNA-mediated interference through ingested double-stranded RNA in Caenorhabditis elegans. Genome Biol. 2001, 2. [Google Scholar] [CrossRef] [Green Version]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sulston, J.; Hodgkin, J. Methods. In The Nematode Caenorhabditis Elegans; Wood, W., Ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1988; pp. 587–606. [Google Scholar]
- Lamitina, T.; Huang, C.G.; Strange, K. Genome-wide RNAi screening identifies protein damage as a regulator of osmoprotective gene expression. Proc. Natl. Acad. Sci. USA 2006, 103, 12173–12178. [Google Scholar] [CrossRef] [Green Version]
- Han, S.K.; Lee, D.; Lee, H.; Kim, D.; Son, H.G.; Yang, J.-S.; Lee, S.-J.V.; Kim, S. OASIS 2: Online application for survival analysis 2 with features for the analysis of maximal lifespan and healthspan in aging research. Oncotarget 2016, 7, 56147–56152. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasulova, M.; Zečić, A.; Monje Moreno, J.M.; Vandemeulebroucke, L.; Dhondt, I.; Braeckman, B.P. Elevated Trehalose Levels in C. elegans daf-2 Mutants Increase Stress Resistance, Not Lifespan. Metabolites 2021, 11, 105. https://doi.org/10.3390/metabo11020105
Rasulova M, Zečić A, Monje Moreno JM, Vandemeulebroucke L, Dhondt I, Braeckman BP. Elevated Trehalose Levels in C. elegans daf-2 Mutants Increase Stress Resistance, Not Lifespan. Metabolites. 2021; 11(2):105. https://doi.org/10.3390/metabo11020105
Chicago/Turabian StyleRasulova, Madina, Aleksandra Zečić, Jose Manuel Monje Moreno, Lieselot Vandemeulebroucke, Ineke Dhondt, and Bart P. Braeckman. 2021. "Elevated Trehalose Levels in C. elegans daf-2 Mutants Increase Stress Resistance, Not Lifespan" Metabolites 11, no. 2: 105. https://doi.org/10.3390/metabo11020105
APA StyleRasulova, M., Zečić, A., Monje Moreno, J. M., Vandemeulebroucke, L., Dhondt, I., & Braeckman, B. P. (2021). Elevated Trehalose Levels in C. elegans daf-2 Mutants Increase Stress Resistance, Not Lifespan. Metabolites, 11(2), 105. https://doi.org/10.3390/metabo11020105