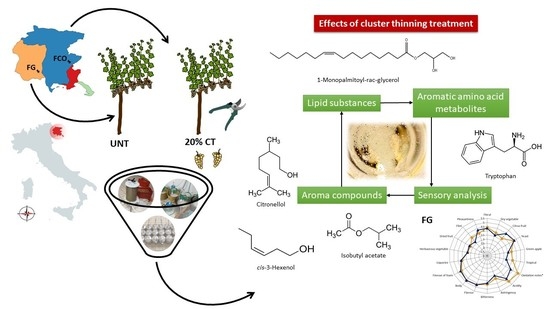
Cluster Thinning and Vineyard Site Modulate the Metabolomic Profile of Ribolla Gialla Base and Sparkling Wines
Abstract
:1. Introduction
2. Results
2.1. Weather Conditions
2.2. Yield and Basic Grape Quality Parameters
2.3. Wine Basic Compositional Parameters
2.4. Volatile Profile of Base Wines and Sparkling Wines
2.5. Lipid Profile of Base Wines and Sparkling Wines
2.6. Aromatic Amino Acid Metabolites Profile in Base Wines and Sparkling Wines
2.7. Sensory Attributes
2.8. Multivariate Analysis of Sparkling Wines
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Vineyard Sites
4.3. Vine Treatments and Harvest of the Grapes
4.4. Vinification of Base Wines
4.5. Secondary Fermentation of Sparkling Wines
4.6. Enological Parameters
4.7. Volatile Compound Analysis
4.8. Lipid Compound Analysis
4.9. Aromatic Amino Acid Metabolites Analysis
4.10. Sensory Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bavčar, D.; Baša Česnik, H.; Čuš, F.; Košmerl, T. The influence of skin contact during alcoholic fermentation on the aroma composition of Ribolla Gialla and Malvasia Istriana Vitis vinifera (L.) grape wines. Int. J. Food Sci. Technol. 2011, 46, 1801–1808. [Google Scholar] [CrossRef]
- Bavčar, D.; Česnik, H.B.; Čuš, F.; Gašperlin, L.; Košmerl, T.; Košmerl, T. Impact of Alternative skin contact procedures on the aroma composition of white wine. S. Afr. J. Enol. Vitic. 2016, 32, 190–203. [Google Scholar] [CrossRef]
- Dashko, S.; Zhou, N.; Tinta, T.; Sivilotti, P.; Lemut, M.S.; Trost, K.; Gamero, A.; Boekhout, T.; Butinar, L.; Vrhovsek, U.; et al. Use of non-conventional yeast improves the wine aroma profile of Ribolla Gialla. J. Ind. Microbiol. Biotechnol. 2015, 42, 997–1010. [Google Scholar] [CrossRef] [PubMed]
- Alem, H.; Rigou, P.; Schneider, R.; Ojeda, H.; Torregrosa, L. Impact of agronomic practices on grape aroma composition: A review. J. Sci. Food Agric. 2019, 99, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, Y.N.; Chen, W.K.; He, F.; Chen, W.; Cai, X.D.; Duan, C.Q.; Wang, J. Effects of cluster thinning on vine photosynthesis, berry ripeness and flavonoid composition of Cabernet Sauvignon. Food Chem. 2018, 248, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Sivilotti, P.; Falchi, R.; Vanderweide, J.; Sabbatini, P.; Bubola, M.; Vanzo, A.; Lisjak, K.; Peterlunger, E.; Herrera, J.C. Yield reduction through cluster or selective berry thinning similarly modulates anthocyanins and proanthocyanidins composition in Refosco dal peduncolo rosso (Vitis vinifera L.) grapes. Sci. Hortic. (Amsterdam) 2020, 264, 109166. [Google Scholar] [CrossRef]
- Avizcuri-Inac, J.M.; Gonzalo-Diago, A.; Sanz-Asensio, J.; Martínez-Soria, M.T.; López-Alonso, M.; Dizy-Soto, M.; Echávarri-Granado, J.F.; Vaquero-Fernández, L.; Fernández-Zurbano, P. Effect of cluster thinning and prohexadione calcium applications on phenolic composition and sensory properties of red wines. J. Agric. Food Chem. 2013, 61, 1124–1137. [Google Scholar] [CrossRef]
- Chapman, D.M.; Matthews, M.A.; Guinard, J.-X. Sensory Attributes of Cabernet Sauvignon wines made from vines with different crop yields. Am. J. Enol. Vitic. 2004, 55, 325–334. [Google Scholar]
- Condurso, C.; Cincotta, F.; Tripodi, G.; Sparacio, A.; Giglio, D.M.L.; Sparla, S.; Verzera, A. Effects of cluster thinning on wine quality of Syrah cultivar (Vitis vinifera L.). Eur. Food Res. Technol. 2016, 242, 1719–1726. [Google Scholar] [CrossRef]
- Rutan, T.E.; Herbst-Johnstone, M.; Kilmartin, P.A. Effect of cluster thinning Vitis vinifera cv. Pinot noir on wine volatile and phenolic composition. J. Agric. Food Chem. 2018, 66, 10053–10066. [Google Scholar] [CrossRef]
- Talaverano, I.; Valdés, E.; Moreno, D.; Gamero, E.; Mancha, L.; Vilanova, M. The combined effect of water status and crop level on Tempranillo wine volatiles. J. Sci. Food Agric. 2017, 97, 1533–1542. [Google Scholar] [CrossRef]
- Bowen, A.J.; Reynolds, A.G. Aroma compounds in Ontario Vidal and Riesling icewines. I. Effects of harvest date. Food Res. Int. 2015, 76, 540–549. [Google Scholar] [CrossRef]
- Bubola, M.; Rusjan, D.; Lukić, I. Crop level vs. leaf removal: Effects on Istrian Malvasia wine aroma and phenolic acids composition. Food Chem. 2020, 312, 126046. [Google Scholar] [CrossRef]
- Moreno Luna, L.H.; Reynolds, A.; Di Profio, F.; Zhang, L.; Kotsaki, E. Crop level and harvest date impact on four Ontario wine grape cultivars. II. Wine aroma compounds and sensory analysis. S. Afr. J. Enol. Vitic. 2018, 39. [Google Scholar] [CrossRef] [Green Version]
- Kliewer, W.M.; Dokoozlian, N.K. Leaf area/crop weight ratios of grapevines: Influence on fruit composition and wine quality. Am. J. Enol. Vitic. 2005, 56, 170–181. [Google Scholar]
- Jones, J.E.; Kerslake, F.L.; Close, D.C.; Dambergs, R.G. Viticulture for sparkling wine production: A review. Am. J. Enol. Vitic. 2014, 65, 407–416. [Google Scholar] [CrossRef] [Green Version]
- Pozo-Bayón, M.A.; Polo, M.C.; Martín-Álvarez, P.J.; Pueyo, E. Effect of vineyard yield on the composition of sparkling wines produced from the grape cultivar Parellada. Food Chem. 2004, 86, 413–419. [Google Scholar] [CrossRef]
- Jackson, D.I.; Lombard, P.B. Environmental and management practices affecting grape composition and wine quality–A review. Am. J. Enol. Vitic. 1993, 44, 409–430. [Google Scholar]
- Tesnière, C. Importance and role of lipids in wine yeast fermentation. Appl. Microbiol. Biotechnol. 2019, 103, 8293–8300. [Google Scholar] [CrossRef]
- Gallart, M.; Francioli, S.; Viu-Marco, A.; López-Tamames, E.; Buxaderas, S. Determination of free fatty acids and their ethyl esters in musts and wines. J. Chromatogr. A 1997, 776, 283–291. [Google Scholar] [CrossRef]
- Gallart, M.; Lopez-tamames, E.; Suberbiola, G.; Buxaderas, S. Influence of fatty acids on wine foaming. J. Agric. Food Chem. 2002, 50, 7042–7045. [Google Scholar] [CrossRef] [PubMed]
- Pueyo, E.; Martín-Alvarez, P.J.; Polo, M.C. Relationship between foam characteristics and chemical composition in wines and cavas (sparkling wines). Am. J. Enol. Vitic. 1995, 46, 518–524. [Google Scholar]
- Álvarez-Fernández, M.A.; Fernández-Cruz, E.; Garcia-Parrilla, M.C.; Troncoso, A.M.; Mattivi, F.; Vrhovsek, U.; Arapitsas, P. Saccharomyces cerevisiae and Torulaspora delbrueckiiintra- and extra-cellular aromatic amino acids metabolism. J. Agric. Food Chem. 2019, 67, 7942–7953. [Google Scholar] [CrossRef] [PubMed]
- Hoenicke, K.; Borchert, O.; Grüning, K.; Simat, T.J. “Untypical aging off-flavor” in wine: Synthesis of potential degradation compounds of indole-3-acetic acid and kynurenine and their evaluation as precursors of 2-aminoacetophenone. J. Agric. Food Chem. 2002, 50, 4303–4309. [Google Scholar] [CrossRef]
- Hoenicke, K.; Simat, T.J.; Steinhart, H.; Köhler, H.J.; Schwab, A. Determination of free and conjugated indole-3-acetic acid, tryptophan, and tryptophan metabolites in grape must and wine. J. Agric. Food Chem. 2001, 49, 5494–5501. [Google Scholar] [CrossRef]
- Arapitsas, P.; Guella, G.; Mattivi, F. The impact of SO2 on wine flavanols and indoles in relation to wine style and age. Sci. Rep. 2018, 8, 858. [Google Scholar] [CrossRef] [Green Version]
- Degano, F.; Cisilino, D.; Bigot, G.; Sivilotti, P.; Paladin, M. Le Stagioni e le uve 2018. Consorzio di Tutela Vini D.O.C. Friuli Colli Orientali & Ramandolo; Grafiche Filacorda: Manzano, Italy, 2018. [Google Scholar]
- Reynolds, A.G.; Yerle, S.; Watson, B.; Price, S.F.; Wardle, D.A. Fruit environment and crop level effects on Pinot noir. III. Composition and descriptive analysis of Oregon and British Columbia wines. Am. J. Enol. Vitic. 1996, 47, 329–339. [Google Scholar]
- Bowen, A.J.; Reynolds, A.G. Aroma compounds in Ontario Vidal and Riesling icewines. II. Effects of crop level. Food Res. Int. 2015, 76, 550–560. [Google Scholar] [CrossRef]
- Voce, S.; Škrab, D.; Vrhovsek, U.; Battistutta, F.; Comuzzo, P.; Sivilotti, P. Compositional characterization of commercial sparkling wines from cv. Ribolla Gialla produced in Friuli Venezia Giulia. Eur. Food Res. Technol. 2019, 245, 2279–2292. [Google Scholar] [CrossRef]
- Bubola, M.; Peršurić, Đ.; Kovačević Ganić, K. Impact of cluster thinning on productive characteristics and wine phenolic composition of cv. Merlot. J. Food Agric. Environ. 2011, 9, 36–39. [Google Scholar]
- Liu, S.-Q.; Pilone, G.J. An overview of formation and roles of acetaldehyde in winemaking with emphasis on microbiological implications. Int. J. Food Sci. Technol. 2000, 35, 49–61. [Google Scholar] [CrossRef]
- Fariña, L.; Villar, V.; Ares, G.; Carrau, F.; Dellacassa, E.; Boido, E. Volatile composition and aroma profile of Uruguayan Tannat wines. Food Res. Int. 2015, 69, 244–255. [Google Scholar] [CrossRef]
- De-La-Fuente-Blanco, A.; Sáenz-Navajas, M.P.; Ferreira, V. On the effects of higher alcohols on red wine aroma. Food Chem. 2016, 210, 107–114. [Google Scholar] [CrossRef]
- Jagatić Korenika, A.-M.; Preiner, D.; Tomaz, I.; Jeromel, A. Volatile profile characterization of Croatian commercial sparkling wines. Molecules 2020, 25, 4349. [Google Scholar] [CrossRef]
- Vilela, A. Modulating wine pleasantness throughout wine-yeast co-inoculation or sequential inoculation. Fermentation 2020, 6, 22. [Google Scholar] [CrossRef] [Green Version]
- Duan, L.L.; Shi, Y.; Jiang, R.; Yang, Q.; Wang, Y.Q.; Liu, P.T.; Duan, C.Q.; Yan, G.L. Effects of adding unsaturated fatty acids composition of Saccharomyces cerevisiae and compounds in wine on fatty acid major volatile. S. Afr. J. Enol. Vitic. 2015, 36, 285–295. [Google Scholar] [CrossRef] [Green Version]
- Santos, L.P.; Morais, D.R.; Souza, N.E.; Cottica, S.M.; Boroski, M.; Visentainer, J.V. Phenolic compounds and fatty acids in different parts of Vitis labrusca and V. vinifera grapes. Food Res. Int. 2011, 44, 1414–1418. [Google Scholar] [CrossRef]
- Hatanaka, A. The biogeneration of green odour by green leaves. Phytochemistry 1993, 34, 1201–1218. [Google Scholar] [CrossRef]
- Kemp, B.; Condé, B.; Jégou, S.; Howell, K.; Vasserot, Y.; Marchal, R. Chemical compounds and mechanisms involved in the formation and stabilization of foam in sparkling wines. Crit. Rev. Food Sci. Nutr. 2019, 59, 2072–2094. [Google Scholar] [CrossRef]
- Coelho, E.; Reis, A.; Domingues, M.R.M.; Rocha, S.M.; Coimbra, M.A. Synergistic effect of high and low molecular weight molecules in the foamability and foam stability of sparkling wines. J. Agric. Food Chem. 2011, 59, 3168–3179. [Google Scholar] [CrossRef]
- Vollhardt, D.; Brezesinski, G. Phase Characteristics of 1-monopalmitoyl-rac-glycerol monolayers at the air/water interface. J. Agric. Food Chem. 2016, 32, 7316–7325. [Google Scholar] [CrossRef] [PubMed]
- Della Corte, A.; Chitarrini, G.; Di Gangi, I.M.; Masuero, D.; Soini, E.; Mattivi, F.; Vrhovsek, U. A rapid LC–MS/MS method for quantitative profiling of fatty acids, sterols, glycerolipids, glycerophospholipids and sphingolipids in grapes. Talanta 2015, 140, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Navarro, J.; Da Ros, A.; Masuero, D.; Izquierdo-Cañas, P.M.; Hermosín-Gutiérrez, I.; Gómez-Alonso, S.; Mattivi, F.; Vrhovsek, U. LC-MS/MS analysis of free fatty acid composition and other lipids in skins and seeds of Vitis vinifera grape cultivars. Food Res. Int. 2019, 125, 108556. [Google Scholar] [CrossRef] [PubMed]
- Pueyo, E.; Martínez-Rodríguez, A.; Carmen Polo, M.; Santa-María, G.; Bartolomé, B. Release of lipids during yeast autolysis in a model wine system. J. Agric. Food Chem. 2000, 48, 116–122. [Google Scholar] [CrossRef]
- Dickey, A.N.; Yim, W.S.; Faller, R. Using ergosterol to mitigate the deleterious effects of ethanol on bilayer structure. J. Phys. Chem. B 2009, 113, 2388–2397. [Google Scholar] [CrossRef]
- Varela, C.; Torrea, D.; Schmidt, S.A.; Ancin-Azpilicueta, C.; Henschke, P.A. Effect of oxygen and lipid supplementation on the volatile composition of chemically defined medium and Chardonnay wine fermented with Saccharomyces cerevisiae. Food Chem. 2012, 135, 2863–2871. [Google Scholar] [CrossRef]
- Álvarez-Fernández, M.A.; Carafa, I.; Vrhovsek, U.; Arapitsas, P. Modulating wine aromatic amino acid catabolites by using Torulaspora delbrueckii in sequentially inoculated fermentations or Saccharomyces cerevisiaealone. Microorganisms 2020, 8, 1349. [Google Scholar] [CrossRef]
- Maslov, L.; Jeromel, A.; Herjavec, S.; Jagatić Korenika, A.-M.; Mihaljević, M.; Plavša, T. Indole-3-acetic acid and tryptophan in Istrian Malvasia grapes and wine. J. Food Agric. Environ. 2005, 99, 29–33. [Google Scholar]
- Mattivi, F.; Vrhovšek, U.; Versini, G. Determination of indole-3-acetic acid, tryptophan and other indoles in must and wine by high-performance liquid chromatography with fluorescence detection. J. Chromatogr. A 1999, 855, 227–235. [Google Scholar] [CrossRef]
- Tudela, R.; Ribas-Agustí, A.; Buxaderas, S.; Riu-Aumatell, M.; Castellari, M.; López-Tamames, E. Ultrahigh-performance liquid chromatography (UHPLC)-tandem mass spectrometry (MS/MS) quantification of nine target indoles in sparkling wines. J. Agric. Food Chem. 2016, 64, 4772–4776. [Google Scholar] [CrossRef]
- Culleré, L.; Escudero, A.; Cacho, J.; Ferreira, V. Gas chromatography-olfactometry and chemical quantitative study of the aroma of six premium quality Spanish aged red wines. J. Agric. Food Chem. 2004, 52, 1653–1660. [Google Scholar] [CrossRef]
- Louw, L.; Tredoux, A.G.J.; Van Rensburg, P.; Kidd, M.; Naes, T.; Nieuwoudt, H.H. Fermentation-derived aroma compounds in varietal young wines from South Africa. S. Afr. J. Enol. Vitic. 2016, 31, 213–225. [Google Scholar] [CrossRef] [Green Version]
- Alessandrini, M.; Gaiotti, F.; Belfiore, N.; Matarese, F.; D’Onofrio, C.; Tomasi, D. Influence of vineyard altitude on Glera grape ripening (Vitis vinifera L.): Effects on aroma evolution and wine sensory profile. J. Sci. Food Agric. 2017, 97, 2695–2705. [Google Scholar] [CrossRef]
- Waterhouse, A.L.; Sacks, G.L.; Jeffery, D.W. Esters. In Understanding Wine Chemistry; John Wiley & Sons, Ltd: Chichester, UK, 2016; pp. 57–67. [Google Scholar]
- Mayr, C.M.; Capone, D.L.; Pardon, K.H.; Black, C.A.; Pomeroy, D.; Francis, I.L. Quantitative analysis by GC-MS/MS of 18 aroma compounds related to oxidative off-flavor in wines. J. Agric. Food Chem. 2015, 63, 3394–3401. [Google Scholar] [CrossRef]
- Carlin, S.; Vrhovsek, U.; Franceschi, P.; Lotti, C.; Bontempo, L.; Camin, F.; Toubiana, D.; Zottele, F.; Toller, G.; Fait, A.; et al. Regional features of northern Italian sparkling wines, identified using solid-phase micro extraction and comprehensive two-dimensional gas chromatography coupled with time-of-flight mass spectrometry. Food Chem. 2016, 208, 68–80. [Google Scholar] [CrossRef]
- Píry, J.; Príbela, A.; Ďurčanská, J.; Farkaš, P. Fractionation of volatiles from blackcurrant (Ribes nigrum L.) by different extractive methods. Food Chem. 1995, 54, 73–77. [Google Scholar] [CrossRef]
- Orav, A.; Kailas, T.; Muurisepp, M. Composition of blackcurrant aroma isolated from leaves, buds, and berries of Ribes nigrum L/Mustsostra (Ribes nigrum L.) lehtede, pungade ja marjade aroomi keemiline koostis. Est. Acad. Sci. Chem. 2002, 51, 225–235. [Google Scholar]
- Schneider, R.; Razungles, A.; Augier, C.; Baumes, R. Monoterpenic and norisoprenoidic glycoconjugates of Vitis vinifera L. cv. Melon, B. as precursors of odorants in Muscadet wines. J. Chromatogr. A 2001, 936, 145–157. [Google Scholar] [CrossRef]
- Ong, P.K.C.; Acree, T.E. Similarities in the aroma chemistry of Gewurztraminer variety wines and lychee (Litchi chinesis Sonn.) fruit. J. Agric. Food Chem. 1999, 47, 665–670. [Google Scholar] [CrossRef]
- López-Tamames, E.; Carro-Mariño, N.; Gunata, Y.Z.; Sapis, C.; Baumes, R.; Bayonove, C. Potential aroma in several varieties of spanish grapes. J. Agric. Food Chem. 1997, 45, 1729–1735. [Google Scholar] [CrossRef]
- Brat, P.; Rega, B.; Alter, P.; Reynes, M.; Brillouet, J.M. Distribution of volatile compounds in the pulp, cloud, and serum of freshly squeezed orange juice. J. Agric. Food Chem. 2003, 51, 3442–3447. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Rouseff, R.L. Characterization of aroma-impact compounds in cold-pressed grapefruit oil using time-intensity GC-olfactometry and GC-MS. Flavour Fragr. J. 2001, 16, 457–463. [Google Scholar] [CrossRef]
- Suárez, M.; Duque, C.; Wintoch, H.; Schreier, P. Glycosidically bound aroma compounds from the pulp and the peelings of lulo fruit (Solanum vestissimum D.). J. Agric. Food Chem. 1991, 39, 1643–1645. [Google Scholar] [CrossRef]
- López, R.; Ferreira, V.; Hernández, P.; Cacho, J.F. Identification of impact odorants of young red wines made with Merlot, Cabernet Sauvignon and Grenache grape varieties: A comparative study. J. Sci. Food Agric. 1999, 79, 1461–1467. [Google Scholar] [CrossRef]
- Chu-Chin, C.; Chi-Tand, H. Gas chromatographic analysis of thermal degradation products of gingerol compounds in steam-distilled oil from ginger (Zingiber officinale Roscoe). J. Chromatogr. A 1987, 387, 499–504. [Google Scholar] [CrossRef]
- Wong, K.C.; Lai, F.Y. Volatile Constituents from the fruits of fourSyzygiumspecies grown in Malaysia. Flavour Fragr. J. 1996, 11, 61–66. [Google Scholar] [CrossRef]
- Shimoda, M.; Wu, Y.; Osajima, Y. Aroma Compounds from aqueous solution of haze (Rhus succedanea) honey determined by adsorptive column chromatography. J. Agric. Food Chem. 1996, 44, 3913–3918. [Google Scholar] [CrossRef]
- Takeoka, G.R.; Jennings, W.; Flath, R.A.; Güntert, M. Nectarine volatiles: Vacuum steam distillation versus headspace sampling. J. Agric. Food Chem. 1988, 36, 553–560. [Google Scholar] [CrossRef]
- Chung, H.Y. Volatile components in crabmeats of Charybdis feriatus. J. Agric. Food Chem. 1999, 47, 2280–2287. [Google Scholar] [CrossRef]
- Charles, M.; Martin, B.; Ginies, C.; Etievant, P.; Coste, G.; Guichard, E. Potent aroma compounds of two red wine vinegars. J. Agric. Food Chem. 2000, 48, 70–77. [Google Scholar] [CrossRef]
- De la Calle García, D.; Reichenbächer, M.; Danzer, K.; Hurlbeck, C.; Bartzsch, C.; Feller, K.-H. Investigations on wine bouquet components by solid-phase microextration -capillary gas chromatography (SPME-GC) using different fibers. J. High Resolut. Chromatogr. 1997, 20, 665–668. [Google Scholar] [CrossRef]
- Umano, K.; Shibamoto, T.; Shoji, A.; Hagi, Y. Volatile constituents of peel of quince fruit, Cydonia oblonga Miller. J. Agric. Food Chem. 1986, 34, 593–596. [Google Scholar] [CrossRef]
- Ferreira, V.; Rapp, A.; Cacho, J.F.; Hastrich, H.; Yavas, I. Fast and quantitative determination of wine flavor compounds using microextraction with freon 113. J. Agric. Food Chem. 1993, 41, 1413–1420. [Google Scholar] [CrossRef]
- Miranda-Lopez, R.; Libbey, L.M.; Watson, B.T.; Mcdaniel, M.R. Odor analysis of Pinot noir wines from grapes of different maturities by a gas chromatography-olfactometry technique (osme). J. Food Sci. 1992, 57, 985–993. [Google Scholar] [CrossRef]
- Takeoka, G.; Buttery, R.G.; Flath, R.A.; Teranishi, R.; Wheeler, E.L.; Wieczorek, R.L.; Guentert, M. Volatile Constituents of Pineapple (Ananas Comosus L. Merr.); ACS: Washington, WA, USA, 1989; pp. 223–237. [Google Scholar]
- Tressl, R.; Friese, L.; Fendesack, F.; Koppler, H. Gas chromatographic-mass spectrometric investigation of hop aroma constituents in beer. J. Agric. Food Chem. 1978, 26, 1422–1426. [Google Scholar] [CrossRef]
- Tatsuka, K.; Suekane, S.; Sakai, Y.; Sumitani, H. Volatile constituents of kiwi fruit flowers: Simultaneous distillation and extraction versus headspace sampling. J. Agric. Food Chem. 1990, 38, 2176–2180. [Google Scholar] [CrossRef]
- Canuti, V.; Conversano, M.; Calzi, M.L.; Heymann, H.; Matthews, M.A.; Ebeler, S.E. Headspace solid-phase microextraction-gas chromatography-mass spectrometry for profiling free volatile compounds in Cabernet Sauvignon grapes and wines. J. Chromatogr. A 2009, 1216, 3012–3022. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, Y.; Li, J.; Fan, W.; Jiang, W. Profile of volatile compounds in 11 brandies by headspace solid-phase microextraction followed by gas chromatography-mass spectrometry. J. Food Sci. 2009, 74, C90–C99. [Google Scholar] [CrossRef]
- Iversen, C.K.; Jakobsen, H.B.; Olsen, C.E. Aroma changes during black currant (Ribes nigrum L.) nectar processing. J. Agric. Food Chem. 1998, 46, 1132–1136. [Google Scholar] [CrossRef]
- Brander, C.F.; Kepner, R.E.; Webb, A.D. Identification of some volatile compounds of wine of Vitis Vinifera cultivar Pinot noir. Am. J. Enol. Vitic. 1980, 31, 69–75. [Google Scholar]
- Câmpeanu, G.; Burcea, M.; Doneanu, C.; Nalmolosanu, I.; Visan, L. GC/MS characterization of the volatiles isolated from the wines obtained from the indigenous cultivar Feteasca Regalã. Analusis 1998, 26, 93–96. [Google Scholar] [CrossRef]
- Selli, S.; Canbas, A.; Cabaroglu, T.; Erten, H.; Lepoutre, J.P.; Gunata, Z. Effect of skin contact on the free and bound aroma compounds of the white wine of Vitis vinifera L. cv Narince. Food Control 2006, 17, 75–82. [Google Scholar] [CrossRef]
- Riu-Aumatell, M.; Castellari, M.; López-Tamames, E.; Galassi, S.; Buxaderas, S. Characterisation of volatile compounds of fruit juices and nectars by HS/SPME and GC/MS. Food Chem. 2004, 87, 627–637. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 1 November 2020).
- Lê, S.; Josse, J.; Husson, F. FactoMineR: A Package for Multivariate Analysis. J. Stat. Soft. 2018, 25, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. Available online: http://www.sthda.com/english/rpkgs/factoextra (accessed on 2 November 2020).
- Wickham, H. ggplot2; Use R! Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-24275-0. [Google Scholar]







| Parameter | Treatment (T) | Site (S) | Year (Y) | Y × T | S × T | Y × S | Y × S × T | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UNT | CT | Sig. F 1 | FG | FCO | Sig. F | 2017 | 2018 | 2019 | Sig. F | |||||
| N° clusters/vine | 32.89 a 2 | 25.20 b | *** | 35.18 a | 22.91 b | *** | 27.13 b | 31.99 a | 28.02 b | ** | ns | ns | ns | ns |
| Cluster weight (g) | 190.67 | 200.20 | ns | 180.45 b | 210.43 a | * | 206.96 a | 198.32 ab | 181.03 b | *** | ns | ns | ns | ns |
| Yield (kg/vine) | 6.40 a | 4.69 b | *** | 6.36 a | 4.73 b | *** | 5.38 b | 6.26 a | 5.00 b | *** | ns | ns | ns | ns |
| Yield (t/ha) | 20.11 a | 14.75 b | *** | 18.92 a | 15.94 b | *** | 16.90 b | 19.66 a | 15.74 b | *** | ns | ns | ns | ns |
| TSS (°Bx) | 17.44 | 17.98 | ns | 17.61 | 17.81 | ns | 18.03 ab | 16.79 b | 18.32 a | *** | ns | ns | ns | ns |
| TA (g/L) 3 | 6.94 | 6.74 | ns | 6.66 | 7.03 | ns | 6.61 | 7.11 | 6.81 | ns | ns | ns | ns | ns |
| pH | 3.25 | 3.27 | ns | 3.30 a | 3.22 b | *** | 3.27 | 3.27 | 3.24 | ns | ns | ns | *** | ns |
| Parameter | Treatment (T) | Site (S) | Year (Y) | Y × T | S × T | Y × S | Y × S × T | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UNT | CT | Sig. F 1 | FG | FCO | Sig. F | 2017 | 2018 | 2019 | Sig. F | |||||
| Alcohol (% v/v) | 10.94 b 2 | 11.41 a | *** | 11.23 | 11.14 | ns | 11.51 a | 10.42 b | 11.62 a | *** | ns | ns | ns | ns |
| Reducing sugars (g/L) | 0.99 b | 1.26 b | * | 1.07 | 1.17 | ns | 0.20 c | 1.85 a | 1.32 b | *** | ns | ns | ns | ns |
| TA (g/L) 3 | 7.50 | 7.36 | ns | 7.21 b | 7.65 a | ** | 7.68 a | 7.10 b | 7.51 ab | ** | ns | ns | ns | ns |
| pH | 3.16 | 3.14 | ns | 3.16 | 3.14 | ns | 3.17 a | 3.15 ab | 3.12 b | * | ** | ns | ns | ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Škrab, D.; Sivilotti, P.; Comuzzo, P.; Voce, S.; Degano, F.; Carlin, S.; Arapitsas, P.; Masuero, D.; Vrhovšek, U. Cluster Thinning and Vineyard Site Modulate the Metabolomic Profile of Ribolla Gialla Base and Sparkling Wines. Metabolites 2021, 11, 331. https://doi.org/10.3390/metabo11050331
Škrab D, Sivilotti P, Comuzzo P, Voce S, Degano F, Carlin S, Arapitsas P, Masuero D, Vrhovšek U. Cluster Thinning and Vineyard Site Modulate the Metabolomic Profile of Ribolla Gialla Base and Sparkling Wines. Metabolites. 2021; 11(5):331. https://doi.org/10.3390/metabo11050331
Chicago/Turabian StyleŠkrab, Domen, Paolo Sivilotti, Piergiorgio Comuzzo, Sabrina Voce, Francesco Degano, Silvia Carlin, Panagiotis Arapitsas, Domenico Masuero, and Urška Vrhovšek. 2021. "Cluster Thinning and Vineyard Site Modulate the Metabolomic Profile of Ribolla Gialla Base and Sparkling Wines" Metabolites 11, no. 5: 331. https://doi.org/10.3390/metabo11050331
APA StyleŠkrab, D., Sivilotti, P., Comuzzo, P., Voce, S., Degano, F., Carlin, S., Arapitsas, P., Masuero, D., & Vrhovšek, U. (2021). Cluster Thinning and Vineyard Site Modulate the Metabolomic Profile of Ribolla Gialla Base and Sparkling Wines. Metabolites, 11(5), 331. https://doi.org/10.3390/metabo11050331