Metabolite Profiling and Classification of Highbush Blueberry Leaves under Different Shade Treatments
Abstract
:1. Introduction
2. Results
2.1. Phenotypic Observation and Leaf Colour Analysis
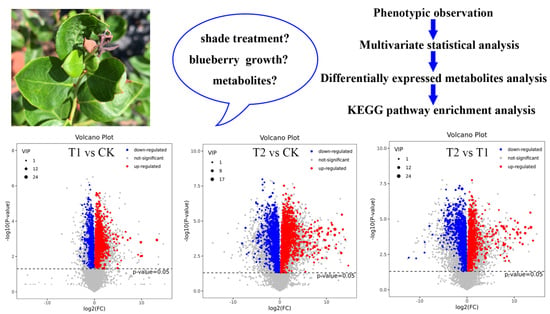
2.2. Multivariate Statistical Analysis
2.3. DEMs Analysis
2.4. KEGG Pathway Enrichment Analysis of DEMs
2.5. Analysis of the Metabolites in Six Pathways
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Determination of Blueberry Leaf Colour
4.3. Sample Preparation
4.4. Sample Processing
4.5. Data Preprocessing
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, J.F.; Kang, F.F.; Yu, A.H.; Yang, W.J.; Chang, E.M.; Jiang, Z.P. Responses of foliar carbohydrates and nutrient status of two distinctive cypress species to shading and nitrogen addition. Glob. Ecol. Conserv. 2018, 16, e00452. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, T.; Fang, S.; Zhou, M.; Qin, J. Responses of morphology, gas exchange, photochemical activity of photosystem II, and antioxidant balance in Cyclocarya paliurus to light spectra. Front. Plant Sci. 2018, 9, 1704. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.J.; D’Souza, C.; Tan, M.Q.; Zhou, W. Light intensity plays contrasting roles in regulating metabolite compositions in choy sum (Brassica rapa var. parachinensis). J. Agric. Food Chem. 2021, 69, 5318–5331. [Google Scholar] [CrossRef] [PubMed]
- Radušienė, J.; Karpavičienė, B.; Stanius, Ž. Effect of external and internal factors on secondary metabolites accumulation in St. John’s worth. Bot. Lith. 2012, 18, 101–108. [Google Scholar] [CrossRef]
- Huang, C.J.; Wei, G.; Jie, Y.C.; Xu, J.J.; Anjum, S.A.; Tanveer, M. Effect of shade on plant traits, gas exchange and chlorophyll content in four ramie cultivars. Photosynthetica 2016, 54, 390–395. [Google Scholar] [CrossRef]
- Jeltsch, F.; Moloney, K.A.; Schurr, F.M.; Köchy, M.; Schwager, M. The state of plant population modelling in light of environmental change. Perspect. Plant Ecol. Evol. Syst. 2008, 9, 171–189. [Google Scholar] [CrossRef]
- Dennis, T.; Li, X.; Xiao, X.; Deng, J.; Ajayo, B.S.; Long, X.; Zhang, Q.; Zhang, X.; Hu, B.; Wang, X.; et al. Spatiotemporal shading regulates anthocyanin, proanthocyanidin, and sucrose accumulation in black soybean seeds. Agron. J. 2020, 112, 708–718. [Google Scholar] [CrossRef]
- Liu, H.; Lin, R.; Deng, X.W. Photobiology: Light signal transduction and photomorphogenesis. J. Integr. Plant Biol. 2020, 62, 1267–1269. [Google Scholar] [CrossRef]
- Xu, P.; Su, H.; Jin, R.; Mao, Y.; Xu, A.; Cheng, H.; Wang, Y.; Meng, Q. Shading effects on leaf color conversion and biosynthesis of the major secondary metabolites in the albino tea cultivar “Yujinxiang”. J. Agric. Food Chem. 2020, 68, 2528–2538. [Google Scholar] [CrossRef]
- Chmura, D.J.; Modrzyński, J.; Chmielarz, P.; Tjoelker, M.G. Plasticity in seedling morphology, biomass allocation and physiology among ten temperate tree species in response to shade is related to shade tolerance and not leaf habit. Plant Biol. 2017, 19, 172–182. [Google Scholar] [CrossRef]
- Cai, Z.Q.; Wang, W.H.; Yang, J.; Cai, C.T. Growth, photosynthesis and root reserpine concentrations of two Rauvolfia species in response to a light gradient. Ind. Crops Prod. 2009, 30, 220–226. [Google Scholar] [CrossRef]
- Szakiel, A.; Pączkowski, C.; Henry, M. Influence of environmental abiotic factors on the content of saponins in plants. Phytochem. Rev. 2011, 10, 471–491. [Google Scholar] [CrossRef]
- Angeletti, P.; Castagnasso, H.; Miceli, E.; Terminiello, L.; Concellón, A.; Chaves, A.; Vicente, A.R. Effect of preharvest calcium applications on postharvest quality, softening and cell wall degradation of two blueberry (Vaccinium corymbosum) varieties. Postharvest Biol. Technol. 2010, 58, 98–103. [Google Scholar] [CrossRef]
- Stevenson, D.; Scalzo, J. Anthocyanin composition and content of blueberries from around the world. J. Berry Res. 2012, 2, 179–189. [Google Scholar] [CrossRef] [Green Version]
- Prior, R.L.; Cao, G.; Martin, A.; Sofic, E.; McEwen, J.; O’Brien, C.; Lischner, N.; Ehlenfeldt, M.; Kalt, W.; Krewer, G.; et al. Antioxidant capacity as influenced by total phenolic and anthocyanin content, maturity, and variety of Vaccinium species. J. Agric. Food Chem. 1998, 46, 2686–2693. [Google Scholar] [CrossRef]
- Bąkowska, A.; Kucharska, A.Z.; Oszmiański, J. The effects of heating, UV irradiation, and storage on stability of the anthocyanin–polyphenol copigment complex. Food Chem. 2003, 81, 349–355. [Google Scholar] [CrossRef]
- Kong, Q.; Wu, A.; Qi, W.; Qi, R.; Carter, J.M.; Rasooly, R.; He, X. Effects of electron-beam irradiation on blueberries inoculated with Escherichia coli and their nutritional quality and shelf life. Postharvest Biol. Technol. 2014, 95, 28–35. [Google Scholar] [CrossRef]
- Chen, L.; Teng, H.; Xie, Z.; Cao, H.; Cheang, W.S.; Skalicka-Woniak, K.; Georgiev, M.I.; Xiao, J. Modifications of dietary flavonoids towards improved bioactivity: An update on structure–activity relationship. Crit. Rev. Food Sci. Nutr. 2017, 58, 513–527. [Google Scholar] [CrossRef]
- Giacalone, M.; Di Sacco, F.; Traupe, I.; Topini, R.; Forfori, F.; Giunta, F. Antioxidant and neuroprotective properties of blueberry polyphenols: A critical review. Nutr. Neurosci. 2011, 14, 119–125. [Google Scholar] [CrossRef]
- Fiehn, O. Metabolomics–The link between genotypes and phenotypes. Plant Mol. Biol. 2002, 48, 155–171. [Google Scholar] [CrossRef]
- Wu, Q.; Zhao, X.; Chen, C.; Zhang, Z.; Yu, F. Metabolite profiling and classification of developing Styrax tonkinensis kernels. Metabolites 2020, 10, 21. [Google Scholar] [CrossRef] [Green Version]
- Bai, Z.Z.; Tang, J.M.; Ni, J.; Zheng, T.T.; Zhou, Y.; Sun, D.Y.; Li, G.N.; Liu, P.; Niu, L.X.; Zhang, Y.L. Comprehensive metabolite profile of multi-bioactive extract from tree peony (Paeonia ostii and Paeonia rockii) fruits based on MS/MS molecular networking. Food Res. Int. 2021, 148, 110609. [Google Scholar] [CrossRef]
- Guo, J.; Wu, Y.; Wang, G.; Wang, T.; Cao, F. Integrated analysis of the transcriptome and metabolome in young and mature leaves of Ginkgo biloba L. Ind. Crops Prod. 2020, 143, 111906. [Google Scholar] [CrossRef]
- Tang, Z.; Fan, Y.; Zhang, L.; Zheng, C.; Chen, A.; Sun, Y.; Guo, H.; Wu, J.; Li, T.; Fan, Y.; et al. Quantitative metabolome and transcriptome analysis reveals complex regulatory pathway underlying photoinduced fiber color formation in cotton. Gene 2021, 767, 145180. [Google Scholar] [CrossRef]
- Rhee, K. Minding the gaps: Metabolomics mends functional genomics. EMBO Rep. 2013, 14, 949–950. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Saito, K.; Yamazaki, M. Integrated omics analysis of specialized metabolism in medicinal plants. Plant J. 2017, 90, 764–787. [Google Scholar] [CrossRef] [PubMed]
- Nakabayashi, R.; Saito, K. Higher dimensional metabolomics using stable isotope labeling for identifying the missing specialized metabolism in plants. Curr. Opin. Plant Biol. 2020, 55, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, H.; Kambe, Y.; Ohshio, M.; Kunihiro, A.; Tanaka, Y.; Suzuki, T.; Nakamura, Y.; Morita, A.; Ikka, T. Integrated metabolome and transcriptome analyses reveal etiolation-induced metabolic changes leading to high amino acid contents in a light-sensitive Japanese albino tea cultivar. Front. Plant Sci. 2021, 11, 611140. [Google Scholar] [CrossRef]
- Carvalho, R.F.; Takaki, M.; Azevedo, R.A. Plant pigments: The many faces of light perception. Acta Physiol. Plant. 2011, 33, 241–248. [Google Scholar] [CrossRef]
- Yusof, F.F.M.; Yaacob, J.S.; Osman, N.; Ibrahim, M.H.; Wan-Mohtar, W.A.A.Q.I.; Berahim, Z.; Zain, N.A.M. Shading effects on leaf gas exchange, leaf pigments and secondary metabolites of polygonum minus huds, an aromatic medicinal herb. Plants 2021, 10, 608. [Google Scholar] [CrossRef]
- Maulidiani, H.; Khatib, A.; Shaari, K.; Abas, F.; Shitan, M.; Kneer, R.; Neto, V.; Lajis, N.H. Discrimination of three Pegaga (Centella) varieties and determination of growth-lighting effects on metabolites content based on the chemometry of 1H nuclear magnetic resonance spectroscopy. J. Agric. Food Chem. 2012, 60, 410–417. [Google Scholar] [CrossRef]
- Kim, S.J.; Yu, D.J.; Kim, T.C.; Lee, H.J. Growth and photosynthetic characteristics of blueberry (Vaccinium corymbosum cv. Bluecrop) under various shade levels. Sci. Hortic. 2011, 129, 486–492. [Google Scholar] [CrossRef]
- Arena, M.E.; Postemsky, P.D.; Curvetto, N.R. Changes in the phenolic compounds and antioxidant capacity of Berberis microphylla G. Forst. berries in relation to light intensity and fertilization. Sci. Hortic. 2017, 218, 63–71. [Google Scholar] [CrossRef]
- Chen, C.; Luo, X.; Jin, G.; Cheng, Z.; Pan, X.; Zhu, G.; Li, S.; Zhu, Y.; Tang, N. Shading effect on survival, growth, and contents of secondary metabolites in micropropagated Anoectochilus plantlets. Braz. J. Bot. 2017, 40, 599–607. [Google Scholar] [CrossRef]
- Rozali, S.E.; Rashid, K.A.; Farzinebrahimi, R. Effects of shading treatments on pigmentation and inflorescence quality of calathea crotalifera bracts. Int. J. Agric. Biol. 2016, 18, 549–556. [Google Scholar] [CrossRef]
- Mathur, S.; Jain, L.; Jajoo, A. Photosynthetic efficiency in sun and shade plants. Photosynthetica 2018, 56, 354–365. [Google Scholar] [CrossRef]
- Park, J.E.; Kim, J.; Purevdorj, E.; Son, Y.J.; Nho, C.W.; Yoo, G. Effects of long light exposure and drought stress on plant growth and glucosinolate production in pak choi (Brassica rapa subsp. chinensis). Food Chem. 2021, 340, 128167. [Google Scholar] [CrossRef]
- Lazzarin, M.; Meisenburg, M.; Meijer, D.; van Ieperen, W.; Marcelis, L.F.M.; Kappers, I.F.; van der Krol, A.R.; van Loon, J.J.A.; Dicke, M. LEDs make it resilient: Effects on plant growth and defense. Trends Plant Sci. 2021, 26, 496–508. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.S.; Choi, J.H.; Son, N.; Kim, S.H.; Park, J.D.; Jang, D.J.; Jeong, Y.; Kim, H.J. Metabolomic analysis of the effect of shade treatment on the nutritional and sensory qualities of green tea. J. Agric. Food Chem. 2013, 61, 332–338. [Google Scholar] [CrossRef]
- Chen, B.L.; Yang, H.K.; Ma, Y.N.; Liu, J.R.; Lv, F.J.; Chen, J.; Meng, Y.L.; Wang, Y.H.; Zhou, Z.G. Effect of shading on yield, fiber quality and physiological characteristics of cotton subtending leaves on different fruiting positions. Photosynthetica 2017, 55, 240–250. [Google Scholar] [CrossRef]
- Jang, I.; Do, G.; Hwang, H.; Suh, S.; Yu, J.; Jang, I.; Moon, J.; Chun, C. Morphological development and photosynthetic acclimation of Panax ginseng seedlings to irradiation by light-emitting diodes (LEDs). Hortic. Environ. Biotechnol. 2021, 62, 571–579. [Google Scholar] [CrossRef]
- Goodacre, R.; Vaidyanathan, S.; Dunn, W.B.; Harrigan, G.G.; Kell, D.B. Metabolomics by numbers: Acquiring and understanding global metabolite data. Trends Biotechnol. 2004, 22, 245–252. [Google Scholar] [CrossRef]
- Tweeddale, H.; Notley-McRobb, L.; Ferenci, T. Effect of slow growth on metabolism of Escherichia coli, as revealed by global metabolite pool (“metabolome”) analysis. J. Bacteriol. 1998, 180, 5109–5116. [Google Scholar] [CrossRef] [Green Version]
- Hamade, K.; Fliniaux, O.; Fontaine, J.X.; Molinié, R.; Otogo Nnang, E.; Bassard, S.; Guénin, S.; Gutierrez, L.; Lainé, E.; Hano, C.; et al. NMR and LC-MS-based metabolomics to study osmotic stress in lignan-deficient flax. Molecules 2021, 26, 767. [Google Scholar] [CrossRef]
- Ackah, M.; Shi, Y.; Wu, M.; Wang, L.; Guo, P.; Guo, L.; Jin, X.; Li, S.; Zhang, Q.; Qiu, C.; et al. Metabolomics response to drought stress in morus alba L. variety Yu-711. Plants 2021, 10, 1636. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Wu, J.; Shahid, M.Q.; He, Y.; Lin, S.; Liu, Z.; Yang, X. Identification of key taste components in loquat using widely targeted metabolomics. Food Chem. 2020, 323, 126822. [Google Scholar] [CrossRef] [PubMed]
- Cortell, J.M.; Kennedy, J.A. Effect of shading on accumulation of flavonoid compounds in (Vitis vinifera L.) pinot noir fruit and extraction in a model system. J. Agric. Food Chem. 2006, 54, 8510–8520. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, M.; Mumm, R.; de Vos, R.C.H.; Ruan, J. Metabolomics reveals the witin-plant spatial effects of shading on tea plants. Tree Physiol. 2021, 41, 317–330. [Google Scholar] [CrossRef]
- Ku, K.M.; Choi, J.N.; Kim, J.; Kim, J.K.; Yoo, L.G.; Lee, S.J.; Hong, Y.-S.; Lee, C.H. Metabolomics analysis reveals the compositional differences of shade grown tea (Camellia sinensis L.). J. Agric. Food Chem. 2009, 58, 418–426. [Google Scholar] [CrossRef]
- Alboresi, A.; Dall’Osto, L.; Aprile, A.; Carillo, P.; Roncaglia, E.; Cattivelli, L.; Bassi, R. Reactive oxygen species and transcript analysis upon excess light treatment in wild-type Arabidopsis thaliana vs a photosensitive mutant lacking zeaxanthin and lutein. BMC Plant Biol. 2011, 11, 62. [Google Scholar] [CrossRef] [Green Version]
- Landi, M.; Tattini, M.; Gould, K. Multiple functional roles of anthocyanins in plant-environment interactions. Environ. Exp. Bot. 2015, 119, 4–17. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, M.; Ruan, J. Metabolomics analysis reveals the metabolic and functional roles of flavonoids in light-sensitive tea leaves. BMC Plant Biol. 2017, 17, 64. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.S.; Gao, L.P.; Shan, Y.; Liu, Y.J.; Tian, Y.W.; Xia, T. Influence of shade on flavonoid biosynthesis in tea (Camellia sinensis (L.) O. Kuntze). Sci. Hortic. 2012, 141, 7–16. [Google Scholar] [CrossRef]
- Karppinen, K.; Zoratti, L.; Nguyenquynh, N.; Häggman, H.; Jaakola, L. On the developmental and environmental regulation of secondary metabolism in vaccinium spp. Berries. Front. Plant Sci. 2016, 7, 655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Fang, S.; Yang, W.; Shang, X.; Fu, X. Light quality affects flavonoid production and related gene expression in Cyclocarya paliurus. J. Photochem. Photobiol. B Biol. 2018, 179, 66–73. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, C.; Huang, Z.; Lyu, L.; Li, J.; Li, W.; Wu, W. The color difference of rubus fruits is closely related to the composition of flavonoids including anthocyanins. LWT 2021, 149, 111825. [Google Scholar] [CrossRef]
- He, Y.; Pan, L.; Yang, T.; Wang, W.; Li, C.; Chen, B.; Shen, Y. Metabolomic and confocal laser scanning microscopy (CLSM) analyses reveal the important function of flavonoids in amygdalus pedunculata pall leaves with temporal changes. Front. Plant Sci. 2021, 12, 648277. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, J.; Yang, F.; Zhou, W.; Mao, S.; Lin, J.; Yan, X. Untargeted LC-MS-based metabolomics revealed specific metabolic changes in cotyledons and roots of Ricinus communis during early seedling establishment under salt stress. Plant Physiol. Biochem. 2021, 163, 108–118. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, X. Transcriptome and metabolomic analyses reveal regulatory networks controlling maize stomatal development in response to blue light. Int. J. Mol. Sci. 2021, 22, 5393. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Liu, Y.; Jiang, W.; Meng, X.; Zhang, W.; Chen, W.; Peng, D.; Xing, S. UPLC/MS-based untargeted metabolomics reveals the changes of metabolites profile of Salvia miltiorrhiza bunge during sweating processing. Sci. Rep. 2020, 10, 19524. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Yang, H.; Huang, Z.; Zhang, C.; Lyu, L.; Li, W.; Wu, W. Metabolite Profiling and Classification of Highbush Blueberry Leaves under Different Shade Treatments. Metabolites 2022, 12, 79. https://doi.org/10.3390/metabo12010079
Wu Y, Yang H, Huang Z, Zhang C, Lyu L, Li W, Wu W. Metabolite Profiling and Classification of Highbush Blueberry Leaves under Different Shade Treatments. Metabolites. 2022; 12(1):79. https://doi.org/10.3390/metabo12010079
Chicago/Turabian StyleWu, Yaqiong, Hao Yang, Zhengjin Huang, Chunhong Zhang, Lianfei Lyu, Weilin Li, and Wenlong Wu. 2022. "Metabolite Profiling and Classification of Highbush Blueberry Leaves under Different Shade Treatments" Metabolites 12, no. 1: 79. https://doi.org/10.3390/metabo12010079
APA StyleWu, Y., Yang, H., Huang, Z., Zhang, C., Lyu, L., Li, W., & Wu, W. (2022). Metabolite Profiling and Classification of Highbush Blueberry Leaves under Different Shade Treatments. Metabolites, 12(1), 79. https://doi.org/10.3390/metabo12010079