The Endogenous Metabolite Glycerophosphocholine Promotes Longevity and Fitness in Caenorhabditis elegans
Abstract
:1. Introduction
2. Results
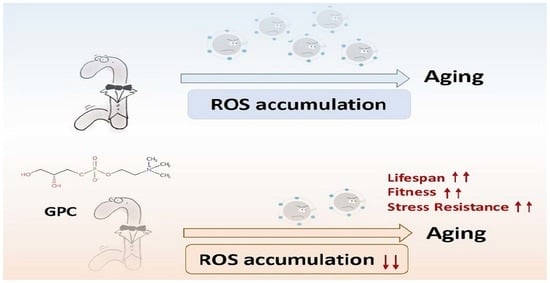
2.1. Endogenous Metabolite GPC Prolongs the Lifespan and Improves the Fitness in C. elegans
2.2. GPC Inhibits the Lipofuscin Accumulation in C. elegans
2.3. GPC has no Adverse Effect on the Fertility and Body Length of C. elegans
2.4. Glycerophosphocholine Enhances Stress Resistance in C. elegans
2.5. GPC Decreases the Intracellular ROS Level in C. elegans
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Metabolite Extraction from Plasma
4.3. UPLC-ESI-MS/MS Analysis
4.4. C. elegans Maintenance
4.5. Lifespan Assay
4.6. Locomotion Behavior Assay and Pharyngeal Pumping Assay
4.7. Lipofuscin Accumulation Assay
4.8. Fecundity Assay
4.9. Body Length Assay
4.10. Stress Assays
4.11. Determination of ROS in C. elegans
4.12. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, S.; Tchkonia, T.; Jiang, J.; Kirkland, J.L.; Sun, Y. Targeting senescent cells for a healthier aging: Challenges and opportunities. Adv. Sci. 2020, 7, 2002611. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Zhang, Y. Targeting autophagy in aging and aging-related cardiovascular diseases. Trends Pharm. Sci. 2018, 39, 1064–1076. [Google Scholar] [CrossRef] [PubMed]
- Villeda, S.A.; Luo, J.; Mosher, K.I.; Zou, B.; Britschgi, M.; Bieri, G.; Stan, T.M.; Fainberg, N.; Ding, Z.; Eggel, A.; et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 2011, 477, 90–94. [Google Scholar] [CrossRef] [Green Version]
- Yousef, H.; Czupalla, C.J.; Lee, D.; Chen, M.B.; Burke, A.N.; Zera, K.A.; Zandstra, J.; Berber, E.; Lehallier, B.; Mathur, V.; et al. Aged blood impairs hippocampal neural precursor activity and activates microglia via brain endothelial cell VCAM1. Nat. Med. 2019, 25, 988–1000. [Google Scholar] [CrossRef] [PubMed]
- Asadi Shahmirzadi, A.; Edgar, D.; Liao, C.Y.; Hsu, Y.M.; Lucanic, M.; Asadi Shahmirzadi, A.; Wiley, C.D.; Gan, G.; Kim, D.E.; Kasler, H.G.; et al. Alpha-Ketoglutarate, an endogenous metabolite, extends lifespan and compresses morbidity in aging mice. Cell Metab. 2020, 32, 447–456.e6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ryu, D.; Wu, Y.; Gariani, K.; Wang, X.; Luan, P.; D’Amico, D.; Ropelle, E.R.; Lutolf, M.P.; Aebersold, R.; et al. NAD+ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science 2016, 352, 1436–1443. [Google Scholar] [CrossRef] [Green Version]
- Mouchiroud, L.; Houtkooper, R.H.; Moullan, N.; Katsyuba, E.; Ryu, D.; Canto, C.; Mottis, A.; Jo, Y.S.; Viswanathan, M.; Schoonjans, K.; et al. The NAD+/Sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signaling. Cell 2013, 154, 430–441. [Google Scholar] [CrossRef] [Green Version]
- Chin, R.M.; Fu, X.; Pai, M.Y.; Vergnes, L.; Hwang, H.; Deng, G.; Diep, S.; Lomenick, B.; Meli, V.S.; Monsalve, G.C.; et al. The metabolite alpha-ketoglutarate extends lifespan by inhibiting ATP synthase and TOR. Nature 2014, 510, 397–401. [Google Scholar] [CrossRef] [Green Version]
- Denzel, M.S.; Storm, N.J.; Gutschmidt, A.; Baddi, R.; Hinze, Y.; Jarosch, E.; Sommer, T.; Hoppe, T.; Antebi, A. Hexosamine pathway metabolites enhance protein quality control and prolong life. Cell 2014, 156, 1167–1178. [Google Scholar] [CrossRef] [Green Version]
- Holmes-McNary, M.Q.; Cheng, W.L.; Mar, M.H.; Fussell, S.; Zeisel, S.H. Choline and choline esters in human and rat milk and in infant formulas. Am. J. Clin. Nutr. 1996, 64, 572–576. [Google Scholar] [CrossRef]
- Saharia, K.; Arya, U.; Kumar, R.; Sahu, R.; Das, C.K.; Gupta, K.; Dwivedi, H.; Subramaniam, J.R. Reserpine modulates neurotransmitter release to extend lifespan and alleviate age-dependent Abeta proteotoxicity in Caenorhabditis elegans. Exp. Gerontol. 2012, 47, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, K.; Okuda, M.; Shibata, S.; Miyaki, S.; Ohkubo, T.; Izu, H.; Fujii, T. The delaying effect of alpha-glycerophosphocholine on senescence, transthyretin deposition, and osteoarthritis in senescence-accelerated mouse prone 8 mice. Biosci. Biotechnol. Biochem. 2018, 82, 647–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narukawa, M.; Kamiyoshihara, A.; Izu, H.; Fujii, T.; Matsubara, K.; Misaka, T. Efficacy of long-term feeding of alpha-Glycerophosphocholine for aging-related phenomena in old mice. Gerontology 2020, 66, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Moreno, H.C.; de Brugada, I.; Carias, D.; Gallo, M. Long-lasting effects of prenatal dietary choline availability on object recognition memory ability in adult rats. Nutr. Neurosci. 2013, 16, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; Yue, Y.; Park, Y. A living model for obesity and aging research: Caenorhabditis elegans. Crit. Rev. Food Sci. Nutr. 2018, 58, 741–754. [Google Scholar] [CrossRef]
- Kaletta, T.; Hengartner, M.O. Finding function in novel targets: C. elegans as a model organism. Nat. Rev. Drug Discov. 2006, 5, 387–398. [Google Scholar] [CrossRef]
- Herndon, L.A.; Schmeissner, P.J.; Dudaronek, J.M.; Brown, P.A.; Listner, K.M.; Sakano, Y.; Paupard, M.C.; Hall, D.H.; Driscoll, M. Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature 2002, 419, 808–814. [Google Scholar] [CrossRef]
- Shi, D.; Xia, X.; Cui, A.; Xiong, Z.; Yan, Y.; Luo, J.; Chen, G.; Zeng, Y.; Cai, D.; Hou, L.; et al. The precursor of PI(3,4,5)P3 alleviates aging by activating daf-18(Pten) and independent of daf-16. Nat. Commun. 2020, 11, 4496. [Google Scholar] [CrossRef]
- Pincus, Z.; Mazer, T.C.; Slack, F.J. Autofluorescence as a measure of senescence in C. elegans: Look to red, not blue or green. Aging 2016, 8, 889–898. [Google Scholar] [CrossRef] [Green Version]
- Brunk, U.T.; Terman, A. Lipofuscin: Mechanisms of age-related accumulation and influence on cell function12 1Guest Editor: Rajindar, S. Sohal 2This article is part of a series of reviews on “Oxidative Stress and Aging.” The full list of papers may be found on the homepage of the journal. Free Radic. Biol. Med. 2002, 33, 611–619. [Google Scholar] [CrossRef]
- Gruber, J.; Tang, S.Y.; Halliwell, B. Evidence for a trade-off between survival and fitness caused by resveratrol treatment of Caenorhabditis elegans. Ann. N. Y. Acad. Sci. 2007, 1100, 530–542. [Google Scholar] [CrossRef] [PubMed]
- Arantes-Oliveira, N.; Berman, J.R.; Kenyon, C. Healthy animals with extreme longevity. Science 2003, 302, 611. [Google Scholar] [CrossRef] [PubMed]
- Lithgow, G.J.; White, T.M.; Melov, S.; Johnson, T.E. Thermotolerance and extended life-span conferred by single-gene mutations and induced by thermal stress. Proc. Natl. Acad. Sci. USA 1995, 92, 7540–7544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuchmenko, E.B.; Petukhov, D.N.; Donchenko, G.V.; Mkhitaryan, L.S.; Tymoshchuk, S.V.; Strutynskaya, N.A.; Vavilova, G.L.; Sagach, V.F. The effect of complexes of precursors and modulators of coenzyme Q biosynthesis on the functional state of heart mitochondria of old rats. Biochem. Mosc. Suppl. Ser. B Biomed. Chem. 2009, 3, 386–392. [Google Scholar] [CrossRef]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [Green Version]
- Brown, M.K.; Evans, J.L.; Luo, Y. Beneficial effects of natural antioxidants EGCG and alpha-lipoic acid on life span and age-dependent behavioral declines in Caenorhabditis elegans. Pharmacol. Biochem. Behav. 2006, 85, 620–628. [Google Scholar] [CrossRef]
- Zhang, L.; Jie, G.; Zhang, J.; Zhao, B. Significant longevity-extending effects of EGCG on Caenorhabditis elegans under stress. Free Radic. Biol. Med. 2009, 46, 414–421. [Google Scholar] [CrossRef]
- Schinzel, R.T.; Higuchi-Sanabria, R.; Shalem, O.; Moehle, E.A.; Webster, B.M.; Joe, L.; Bar-Ziv, R.; Frankino, P.A.; Durieux, J.; Pender, C.; et al. The Hyaluronidase, TMEM2, Promotes ER Homeostasis and Longevity Independent of the UPR(ER). Cell 2019, 179, 1306–1318.e18. [Google Scholar] [CrossRef]
- Pluvinage, J.V.; Haney, M.S.; Smith, B.A.H.; Sun, J.; Iram, T.; Bonanno, L.; Li, L.; Lee, D.P.; Morgens, D.W.; Yang, A.C.; et al. CD22 blockade restores homeostatic microglial phagocytosis in ageing brains. Nature 2019, 568, 187–192. [Google Scholar] [CrossRef]
- Smith, L.K.; He, Y.; Park, J.S.; Bieri, G.; Snethlage, C.E.; Lin, K.; Gontier, G.; Wabl, R.; Plambeck, K.E.; Udeochu, J.; et al. beta2-microglobulin is a systemic pro-aging factor that impairs cognitive function and neurogenesis. Nat. Med. 2015, 21, 932–937. [Google Scholar] [CrossRef] [Green Version]
- Paban, V.; Fauvelle, F.; Alescio-Lautier, B. Age-related changes in metabolic profiles of rat hippocampus and cortices. Eur. J. Neurosci. 2010, 31, 1063–1073. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Larson, M.G.; McCabe, E.L.; Murabito, J.M.; Rhee, E.P.; Ho, J.E.; Jacques, P.F.; Ghorbani, A.; Magnusson, M.; Souza, A.L.; et al. Distinct metabolomic signatures are associated with longevity in humans. Nat. Commun. 2015, 6, 6791. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, X.; Liu, F.; Wang, X.; Zhang, X.; He, K.; Wang, H. Comprehensive Metabolomic Analysis Reveals Dynamic Metabolic Reprogramming in Hep3B Cells with Aflatoxin B1 Exposure. Toxins 2021, 13, 384. [Google Scholar] [CrossRef] [PubMed]
- Alavez, S.; Vantipalli, M.C.; Zucker, D.J.; Klang, I.M.; Lithgow, G.J. Amyloid-binding compounds maintain protein homeostasis during ageing and extend lifespan. Nature 2011, 472, 226–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Lv, T.; Li, M.; Xue, T.; Liu, H.; Zhang, W.; Ding, X.; Zhuang, Z. Anti-aging effect of polysaccharide from Bletilla striata on nematode Caenorhabditis elegans. Pharmacogn Mag. 2015, 11, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, M.; Dai, Y.; Sun, Y.; Aman, Y.; Xu, Y.; Yu, P.; Zheng, Y.; Yang, J.; Zhu, X. Spermidine inhibits neurodegeneration and delays aging via the PINK1-PDR1-dependent mitophagy pathway in C. elegans. Aging 2020, 12, 16852–16866. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.A.; Shukitt-Hale, B.; Kalt, W.; Ingram, D.K.; Joseph, J.A.; Wolkow, C.A. Blueberry polyphenols increase lifespan and thermotolerance in Caenorhabditis elegans. Aging Cell 2006, 5, 59–68. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Zheng, B.; Deng, N.; Wang, H.; Li, T.; Liu, R.H. Effects of ethyl acetate fractional extract from Portulaca oleracea L. (PO-EA) on lifespan and healthspan in Caenorhabditis elegans. J. Food Sci. 2020, 85, 4367–4376. [Google Scholar] [CrossRef]
- Wu, X.; Tong, Z.H.; Li, L.L.; Yu, H.Q. Toxic effects of imidazolium-based ionic liquids on Caenorhabditis elegans: The role of reactive oxygen species. Chemosphere 2013, 93, 2399–2404. [Google Scholar] [CrossRef]






| Treatment | Total Number of Nematodes | Mean Lifespan (Days) | Maximum Lifespan (Days) | Mean Fold Increase (%) |
|---|---|---|---|---|
| H2O | 98 | 22.02 ± 4.83 | 30.70 ± 1.27 | |
| 10 mM GPC | 113 | 23.47 ± 5.67 * | 34.00 ± 2.70 ** | 6.58 |
| 50 mM GPC | 110 | 26.96 ± 6.71 *** | 39.18 ± 1.80 *** | 22.43 |
| Treatment | Total Number of Nematodes | Mean Lifespan (Days) | Maximum Lifespan (Days) | Mean Fold Increase (%) |
|---|---|---|---|---|
| H2O | 94 | 17.36 ± 3.30 | 22.56 ± 0.83 | |
| 50 mM GPC | 90 | 18.46 ± 3.88 * | 23.67 ± 0.47 ** | 6.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.-Y.; Zheng, R.-Q.; Wang, Y.; Liu, Y.-H.; Jiang, S.; Wang, X.-Z.; He, K.; Pan, X.; Zhou, T.; Li, T.; et al. The Endogenous Metabolite Glycerophosphocholine Promotes Longevity and Fitness in Caenorhabditis elegans. Metabolites 2022, 12, 177. https://doi.org/10.3390/metabo12020177
Liu J-Y, Zheng R-Q, Wang Y, Liu Y-H, Jiang S, Wang X-Z, He K, Pan X, Zhou T, Li T, et al. The Endogenous Metabolite Glycerophosphocholine Promotes Longevity and Fitness in Caenorhabditis elegans. Metabolites. 2022; 12(2):177. https://doi.org/10.3390/metabo12020177
Chicago/Turabian StyleLiu, Jia-Yu, Run-Qi Zheng, Yao Wang, Yan-Hong Liu, Shuai Jiang, Xin-Zheng Wang, Kun He, Xin Pan, Tao Zhou, Tao Li, and et al. 2022. "The Endogenous Metabolite Glycerophosphocholine Promotes Longevity and Fitness in Caenorhabditis elegans" Metabolites 12, no. 2: 177. https://doi.org/10.3390/metabo12020177
APA StyleLiu, J. -Y., Zheng, R. -Q., Wang, Y., Liu, Y. -H., Jiang, S., Wang, X. -Z., He, K., Pan, X., Zhou, T., Li, T., Xia, Q., & Zhang, W. -N. (2022). The Endogenous Metabolite Glycerophosphocholine Promotes Longevity and Fitness in Caenorhabditis elegans. Metabolites, 12(2), 177. https://doi.org/10.3390/metabo12020177