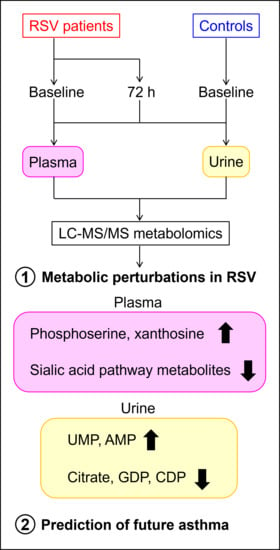
Combined Plasma and Urinary Metabolomics Uncover Metabolic Perturbations Associated with Severe Respiratory Syncytial Viral Infection and Future Development of Asthma in Infant Patients
Abstract
:1. Introduction
2. Results
2.1. Patient Demographics
2.2. Principal Components Analysis Reveals Sample Clustering by RSV Severity
2.3. Plasma Sialic Acid Metabolic Intermediates and Urinary Nucleotides Discriminate RSV Samples
2.4. Patient Weight and Plasma Protein Levels Negatively Correlate with RSV Severity
2.5. Urinary Metabolites Are Predictive of Future Asthma Development
3. Discussion
4. Materials and Methods
4.1. Study Population, Site and Sample Collection
4.2. Metabolite Extraction
4.3. Metabolite Analysis by LC-MS/MS
4.4. Data Processing and Analysis
4.5. Clinical Covariates
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
Appendix A
| Value | Value Type | Categorical to Numeric Conversion |
|---|---|---|
| Asthma classification | categorical | no (0), yes (1) |
| Diet | categorical | breast-fed (0), mixed diet (1), formula-fed (2) |
| ENT 1 problems | categorical | no (0), yes (1) |
| Gender | categorical | male (1), female (2) |
| Prematurity | categorical | no (0), yes (1) |
| Race | categorical | White (1), Other (2) |
| RSV severity | categorical | control (0), moderate (1), severe (2) |
| Sample timepoint | categorical | baseline (0), 72 h (1) |
| Age | numeric (weeks) | |
| Birth weight | numeric (kg) | |
| Eosinophils | numeric (count) | |
| Length of stay | numeric (h) | |
| Length on ventilator | numeric (h) | |
| Lymphocytes | numeric (count) | |
| Neutrophils | numeric (count) | |
| Plasma protein | numeric (µg/mL) | |
| Urine protein | numeric (µg/mL) | |
| WBC 2 | numeric (count) | |
| Weight at hospitalization | numeric (kg) |






References
- Scheltema, N.M.; Gentile, A.; Lucion, F.; Nokes, D.J.; Munywoki, P.K.; Madhi, S.A.; Groome, M.J.; Cohen, C.; Moyes, J.; Thorburn, K.; et al. Global Respiratory Syncytial Virus-Associated Mortality in Young Children (RSV GOLD): A Retrospective Case Series. Lancet Glob. Health 2017, 5, e984–e991. [Google Scholar] [CrossRef] [Green Version]
- Leader, S.; Kohlhase, K. Respiratory Syncytial Virus-Coded Pediatric Hospitalizations, 1997 to 1999. Pediatr. Infect. Dis. J. 2002, 21, 629–632. [Google Scholar] [CrossRef]
- Hall, C.B.; Weinberg, G.A.; Iwane, M.K.; Blumkin, A.K.; Edwards, K.M.; Staat, M.A.; Auinger, P.; Griffin, M.R.; Poehling, K.A.; Erdman, D.; et al. The Burden of Respiratory Syncytial Virus Infection in Young Children. N. Engl. J. Med. 2009, 360, 588–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Priante, E.; Cavicchiolo, M.E.; Baraldi, E. RSV Infection and Respiratory Sequelae. Minerva Pediatr. 2018, 70, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Leimanis, M.L.; Adams, M.; Bachmann, A.S.; Uhl, K.L.; Bupp, C.P.; Hartog, N.L.; Kort, E.J.; Olivero, R.; Comstock, S.S.; et al. Balancing Precision versus Cohort Transcriptomic Analysis of Acute and Recovery Phase of Viral Bronchiolitis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 320, L1147–L1157. [Google Scholar] [CrossRef]
- Shommu, N.S.; Jenne, C.N.; Blackwood, J.; Martin, D.-A.; Joffe, A.R.; Eccles, R.; Brindle, M.; Khanafer, I.; Vogel, H.J.; Thompson, G.C. The Use of Metabolomics and Inflammatory Mediator Profiling Provides a Novel Approach to Identifying Pediatric Appendicitis in the Emergency Department. Sci. Rep. 2018, 8, 4083. [Google Scholar] [CrossRef]
- Daniluk, U.; Daniluk, J.; Kucharski, R.; Kowalczyk, T.; Pietrowska, K.; Samczuk, P.; Filimoniuk, A.; Kretowski, A.; Lebensztejn, D.; Ciborowski, M. Untargeted Metabolomics and Inflammatory Markers Profiling in Children With Crohn’s Disease and Ulcerative Colitis—A Preliminary Study. Inflamm. Bowel Dis. 2019, 25, 1120–1128. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Lou, B.; Liu, J.; She, J. Serum Metabolite Profiles as Potential Biochemical Markers in Young Adults with Community-Acquired Pneumonia Cured by Moxifloxacin Therapy. Sci. Rep. 2020, 10, 4436. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, W.; White, I.R.; Wilkinson, M.; Johnson, C.F.; Rattray, N.; Kishore, A.K.; Goodacre, R.; Smith, C.J.; Fowler, S.J. Breath and Plasma Metabolomics to Assess Inflammation in Acute Stroke. Sci. Rep. 2021, 11, 21949. [Google Scholar] [CrossRef]
- Chen, R.; Mias, G.I.; Li-Pook-Than, J.; Jiang, L.; Lam, H.Y.K.; Chen, R.; Miriami, E.; Karczewski, K.J.; Hariharan, M.; Dewey, F.E.; et al. Personal Omics Profiling Reveals Dynamic Molecular and Medical Phenotypes. Cell 2012, 148, 1293–1307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Morató, J.; Pozo, Ó.J.; Marcos, J. Targeting Human Urinary Metabolome by LC–MS/MS: A Review. Bioanalysis 2018, 10, 489–516. [Google Scholar] [CrossRef]
- Stewart, C.J.; Mansbach, J.M.; Ajami, N.J.; Petrosino, J.F.; Zhu, Z.; Liang, L.; Camargo, C.A.; Hasegawa, K. Serum Metabolome Is Associated with the Nasopharyngeal Microbiota and Disease Severity among Infants with Bronchiolitis. J. Infect. Dis. 2019, 219, 2005–2014. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, L.; Johnson, M.; Mandal, R.; Wishart, D.S. Comprehensive Targeted Metabolomic Assay for Urine Analysis. Anal. Chem. 2020, 92, 10627–10634. [Google Scholar] [CrossRef]
- Roberts, L.D.; Souza, A.L.; Gerszten, R.E.; Clish, C.B. Targeted Metabolomics. Curr. Protoc. Mol. Biol. 2012, 98, 30.2.1–30.2.24. [Google Scholar] [CrossRef] [PubMed]
- Schwaiger-Haber, M.; Stancliffe, E.; Arends, V.; Thyagarajan, B.; Sindelar, M.; Patti, G.J. A Workflow to Perform Targeted Metabolomics at the Untargeted Scale on a Triple Quadrupole Mass Spectrometer. ACS Meas. Sci. Au 2021, 1, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Lunt, S.Y.; Muralidhar, V.; Hosios, A.M.; Israelsen, W.J.; Gui, D.Y.; Newhouse, L.; Ogrodzinski, M.; Hecht, V.; Xu, K.; Acevedo, P.N.M.; et al. Pyruvate Kinase Isoform Expression Alters Nucleotide Synthesis to Impact Cell Proliferation. Mol. Cell 2015, 57, 95–107. [Google Scholar] [CrossRef] [Green Version]
- Teoh, S.T.; Ogrodzinski, M.P.; Ross, C.; Hunter, K.W.; Lunt, S.Y. Sialic Acid Metabolism: A Key Player in Breast Cancer Metastasis Revealed by Metabolomics. Front. Oncol. 2018, 8, 174. [Google Scholar] [CrossRef]
- Yu, L.; Teoh, S.T.; Ensink, E.; Ogrodzinski, M.P.; Yang, C.; Vazquez, A.I.; Lunt, S.Y. Cysteine Catabolism and the Serine Biosynthesis Pathway Support Pyruvate Production during Pyruvate Kinase Knockdown in Pancreatic Cancer Cells. Cancer Metab. 2019, 7, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogrodzinski, M.P.; Teoh, S.T.; Lunt, S.Y. Targeting Subtype-Specific Metabolic Preferences in Nucleotide Biosynthesis Inhibits Tumor Growth in a Breast Cancer Model. Cancer Res. 2021, 81, 303–314. [Google Scholar] [CrossRef]
- Evans, E.D.; Duvallet, C.; Chu, N.D.; Oberst, M.K.; Murphy, M.A.; Rockafellow, I.; Sontag, D.; Alm, E.J. Predicting Human Health from Biofluid-Based Metabolomics Using Machine Learning. Sci. Rep. 2020, 10, 17635. [Google Scholar] [CrossRef]
- Wittig, H.J.; Glaser, J. The Relationship between Bronchiolitis and Childhood Asthma; a Follow-up Study of 100 Cases of Bronchiolitis. J. Allergy 1959, 30, 19–23. [Google Scholar] [CrossRef]
- Stein, R.T.; Sherrill, D.; Morgan, W.J.; Holberg, C.J.; Halonen, M.; Taussig, L.M.; Wright, A.L.; Martinez, F.D. Respiratory Syncytial Virus in Early Life and Risk of Wheeze and Allergy by Age 13 Years. Lancet Lond. Engl. 1999, 354, 541–545. [Google Scholar] [CrossRef]
- Holt, P.G.; Sly, P.D. Viral Infections and Atopy in Asthma Pathogenesis: New Rationales for Asthma Prevention and Treatment. Nat. Med. 2012, 18, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Di Cicco, M.; D’Elios, S.; Peroni, D.G.; Comberiati, P. The Role of Atopy in Asthma Development and Persistence. Curr. Opin. Allergy Clin. Immunol. 2020, 20, 131–137. [Google Scholar] [CrossRef]
- Leteurtre, S.; Duhamel, A.; Grandbastien, B.; Proulx, F.; Cotting, J.; Gottesman, R.; Joffe, A.; Wagner, B.; Hubert, P.; Martinot, A.; et al. Daily Estimation of the Severity of Multiple Organ Dysfunction Syndrome in Critically Ill Children. CMAJ Can. Med. Assoc. J. J. Assoc. Med. Can. 2010, 182, 1181–1187. [Google Scholar] [CrossRef] [Green Version]
- Slater, A.; Shann, F.; Pearson, G. Paediatric Index of Mortality (PIM) Study Group PIM2: A Revised Version of the Paediatric Index of Mortality. Intensive Care Med. 2003, 29, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Pollack, M.M.; Patel, K.M.; Ruttimann, U.E. PRISM III: An Updated Pediatric Risk of Mortality Score. Crit. Care Med. 1996, 24, 743–752. [Google Scholar] [CrossRef]
- Adamko, D.J.; Saude, E.; Bear, M.; Regush, S.; Robinson, J.L. Urine Metabolomic Profiling of Children with Respiratory Tract Infections in the Emergency Department: A Pilot Study. BMC Infect. Dis. 2016, 16, 439. [Google Scholar] [CrossRef] [Green Version]
- Turi, K.N.; Romick-Rosendale, L.; Gebretsadik, T.; Watanabe, M.; Brunwasser, S.; Anderson, L.J.; Moore, M.L.; Larkin, E.K.; Peebles, R.S.; Hartert, T.V. Using Urine Metabolomics to Understand the Pathogenesis of Infant Respiratory Syncytial Virus (RSV) Infection and Its Role in Childhood Wheezing. Metabolomics 2018, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Shukla, A.; Chaudhary, S.K.; Santhosh, R.; Jeyakanthan, J.; Sekar, K. Structural Studies of a Hyperthermophilic Thymidylate Kinase Enzyme Reveal Conformational Substates along the Reaction Coordinate. FEBS J. 2017, 284, 2527–2544. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, N.C.W.; Huesa, C.; Rutsch, F.; MacRae, V.E. New Insights into NPP1 Function: Lessons from Clinical and Animal Studies. Bone 2012, 51, 961–968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torrecilla, A.; Marques, A.F.P.; Buscalioni, R.D.; Oliveira, J.M.A.; Teixeira, N.A.; Atencia, E.A.; Sillero, M.A.G.; Sillero, A. Metabolic Fate of AMP, IMP, GMP and XMP in the Cytosol of Rat Brain: An Experimental and Theoretical Analysis. J. Neurochem. 2001, 76, 1291–1307. [Google Scholar] [CrossRef]
- Zimmermann, A.G.; Gu, J.J.; Laliberté, J.; Mitchell, B.S. Inosine-5′-Monophosphate Dehydrogenase: Regulation of Expression and Role in Cellular Proliferation and T Lymphocyte Activation. Prog. Nucleic Acid Res. Mol. Biol. 1998, 61, 181–209. [Google Scholar] [CrossRef]
- Calise, S.J.; Abboud, G.; Kasahara, H.; Morel, L.; Chan, E.K.L. Immune Response-Dependent Assembly of IMP Dehydrogenase Filaments. Front. Immunol. 2018, 9, 2789. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Hartert, T.V. Evidence for a Causal Relationship between Respiratory Syncytial Virus Infection and Asthma. Expert Rev. Anti-Infect. Ther. 2011, 9, 731–745. [Google Scholar] [CrossRef] [Green Version]
- Lemanske, R.F. The Childhood Origins of Asthma (COAST) Study. Pediatr. Allergy Immunol. 2002, 13, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Matsumoto, K.; Hashimoto, N.; Saikusa, M.; Homma, T.; Yoshihara, S.; Saito, H. Effect of Th1/Th2 Cytokine Pretreatment on RSV-Induced Gene Expression in Airway Epithelial Cells. Int. Arch. Allergy Immunol. 2011, 154, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Clasquin, M.F.; Melamud, E.; Rabinowitz, J.D.; Clasquin, M.F.; Melamud, E.; Rabinowitz, J.D. LC-MS Data Processing with MAVEN: A Metabolomic Analysis and Visualization Engine. In Current Protocols in Bioinformatics, Current Protocols in Bioinformatics; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 14.11.1–14.11.23. ISBN 978-0-471-25095-1. [Google Scholar]
- Dieterle, F.; Ross, A.; Schlotterbeck, G.; Senn, H. Probabilistic Quotient Normalization as Robust Method to Account for Dilution of Complex Biological Mixtures. Application in 1H NMR Metabonomics. Anal. Chem. 2006, 78, 4281–4290. [Google Scholar] [CrossRef]
- Thévenot, E.A.; Roux, A.; Xu, Y.; Ezan, E.; Junot, C. Analysis of the Human Adult Urinary Metabolome Variations with Age, Body Mass Index, and Gender by Implementing a Comprehensive Workflow for Univariate and OPLS Statistical Analyses. J. Proteome Res. 2015, 14, 3322–3335. [Google Scholar] [CrossRef] [PubMed]
- The National Assessment of Educational Progress (NAEP); Third Expert Panel on the Diagnosis and Management of Asthma. Section 3, Component 1: Measures of Asthma Assessment and Monitoring; National Heart, Lung, and Blood Institute (US): Bethesda, MD, USA, 2007.





| Range | (n) | % | Mean | Median | St. dev. | |
|---|---|---|---|---|---|---|
| Age (months) | 0.5–7 | 30 | 100 | 2.24 | 2.0 | 1.60 |
| Controls | 0.5–6 | 10 | 3.20 | 3.0 | 1.83 | |
| RSV | 0–7 | 20 | 2.00 | 2.0 | 1.52 | |
| Gender | ||||||
| Female | 17 | 56.7 | ||||
| Controls | 4 | 13.3 | ||||
| RSV | 13 | 43.3 | ||||
| Birth weight (kg) | ||||||
| Controls | 10 | 3.65 | 3.7 | 0.40 | ||
| RSV | 20 | 3.10 | 3.2 | 0.84 | ||
| Hospital LOS 1 (days) | 20 | 14.93 | 11.20 | 9.37 | ||
| Severe | 15 | 15.73 | 14.58 | 8.63 | ||
| Moderate | 5 | 12.53 | 6.72 | 12.13 | ||
| PICU 2 LOS 1 (days) | 20 | 10.60 | 10.04 | 5.56 | ||
| Severe | 15 | 11.28 | 11.41 | 4.86 | ||
| Moderate | 5 | 8.56 | 5.53 | 7.58 | ||
| Respiratory Support (days) | ||||||
| Mechanical ventilation | 15 | 8.95 | 7.08 | 4.30 | ||
| Mechanical ventilation (non-invs.) | 1 | 2.00 | 2.0 | N/A | ||
| High flow oxygen | 4 | 0.91 | 0.19 | 1.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teoh, S.T.; Leimanis-Laurens, M.L.; Comstock, S.S.; Winters, J.W.; Vandenbosch, N.L.; Prokop, J.W.; Bachmann, A.S.; Lunt, S.Y.; Rajasekaran, S. Combined Plasma and Urinary Metabolomics Uncover Metabolic Perturbations Associated with Severe Respiratory Syncytial Viral Infection and Future Development of Asthma in Infant Patients. Metabolites 2022, 12, 178. https://doi.org/10.3390/metabo12020178
Teoh ST, Leimanis-Laurens ML, Comstock SS, Winters JW, Vandenbosch NL, Prokop JW, Bachmann AS, Lunt SY, Rajasekaran S. Combined Plasma and Urinary Metabolomics Uncover Metabolic Perturbations Associated with Severe Respiratory Syncytial Viral Infection and Future Development of Asthma in Infant Patients. Metabolites. 2022; 12(2):178. https://doi.org/10.3390/metabo12020178
Chicago/Turabian StyleTeoh, Shao Thing, Mara L. Leimanis-Laurens, Sarah S. Comstock, John W. Winters, Nikita L. Vandenbosch, Jeremy W. Prokop, André S. Bachmann, Sophia Y. Lunt, and Surender Rajasekaran. 2022. "Combined Plasma and Urinary Metabolomics Uncover Metabolic Perturbations Associated with Severe Respiratory Syncytial Viral Infection and Future Development of Asthma in Infant Patients" Metabolites 12, no. 2: 178. https://doi.org/10.3390/metabo12020178
APA StyleTeoh, S. T., Leimanis-Laurens, M. L., Comstock, S. S., Winters, J. W., Vandenbosch, N. L., Prokop, J. W., Bachmann, A. S., Lunt, S. Y., & Rajasekaran, S. (2022). Combined Plasma and Urinary Metabolomics Uncover Metabolic Perturbations Associated with Severe Respiratory Syncytial Viral Infection and Future Development of Asthma in Infant Patients. Metabolites, 12(2), 178. https://doi.org/10.3390/metabo12020178