Measurement of the Effect of Accelerated Aging on the Aromatic Compounds of Gewürztraminer and Teroldego Wines, Using a SPE-GC-MS/MS Protocol
Abstract
:1. Introduction
2. Results and Discussion
2.1. Extraction and GC-MS/MS Method Optimization
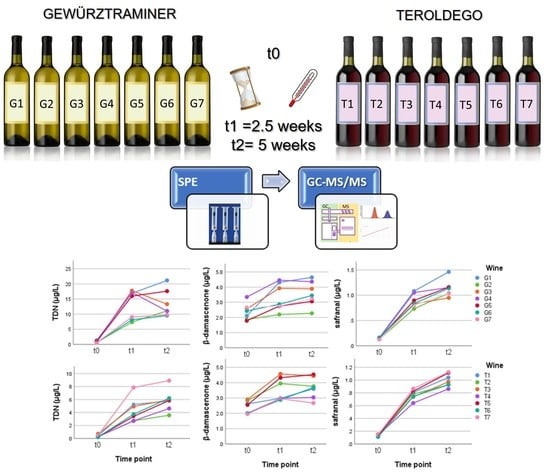
2.2. Accelerated Aging
2.3. Gewürztraminer Wine
2.4. Teroldego Wines
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Wine Samples
3.3. Wine Aging
3.4. Sample Preparation and Extraction
3.5. MS-MS Optimization
3.6. GC-MS/MS Analysis
3.7. Method Validation
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arapitsas, P.; Speri, G.; Angeli, A.; Perenzoni, D.; Mattivi, F. The Influence of Storage on the “Chemical Age” of Red Wines. Metabolomics 2014, 10, 816–832. [Google Scholar] [CrossRef]
- Arapitsas, P.; Dalledonne, S.; Scholz, M.; Catapano, A.; Carlin, S.; Mattivi, F. White Wine Light-Strike Fault: A Comparison between Flint and Green Glass Bottles under the Typical Supermarket Conditions. Food Packag. Shelf Life 2020, 24, 100492. [Google Scholar] [CrossRef]
- Culleré, L.; López, R.; Ferreira, V. Chapter 20—The Instrumental Analysis of Aroma-Active Compounds for Explaining the Flavor of Red Wines. In Red Wine Technology; Morata, A., Ed.; Academic Press: Madrid, Spain, 2019; pp. 283–307. ISBN 978-0-12-814399-5. [Google Scholar]
- Slaghenaufi, D.; Ugliano, M. Norisoprenoids, Sesquiterpenes and Terpenoids Content of Valpolicella Wines During Aging: Investigating Aroma Potential in Relationship to Evolution of Tobacco and Balsamic Aroma in Aged Wine. Front. Chem. 2018, 6. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, V.; Lopez, R. The Actual and Potential Aroma of Winemaking Grapes. Biomolecules 2019, 9, 818. [Google Scholar] [CrossRef] [Green Version]
- Cejudo-Bastante, M.J.; Hermosín-Gutiérrez, I.; Pérez-Coello, M.S. Accelerated Aging against Conventional Storage: Effects on the Volatile Composition of Chardonnay White Wines. J. Food Sci. 2013, 78, C507–C513. [Google Scholar] [CrossRef] [PubMed]
- Voirin, S.G.; Baumes, R.L.; Sapis, J.-C.; Bayonove, C.L. Analytical Methods for Monoterpene Glycosides in Grape and Wine. J. Chromatogr. A 1992, 595, 269–281. [Google Scholar] [CrossRef]
- Boido, E.; Lloret, A.; Medina, K.; Farina, L.; Carrau, F.; Versini, G.; Dellacassa, E. Aroma Composition of Vitis Vinifera Cv. Tannat: The Typical Red Wine from Uruguay. J. Agric. Food Chem. 2003, 51, 5408–5413. [Google Scholar] [CrossRef] [PubMed]
- López, R.; Aznar, M.; Cacho, J.; Ferreira, V. Determination of Minor and Trace Volatile Compounds in Wine by Solid-Phase Extraction and Gas Chromatography with Mass Spectrometric Detection. J. Chromatogr. A 2002, 966, 167–177. [Google Scholar] [CrossRef]
- Paolini, M.; Tonidandel, L.; Moser, S.; Larcher, R. Development of a Fast Gas Chromatography–Tandem Mass Spectrometry Method for Volatile Aromatic Compound Analysis in Oenological Products. J. Mass Spectrom. 2018, 53, 801–810. [Google Scholar] [CrossRef]
- Andujar-Ortiz, I.; Moreno-Arribas, M.V.; Martín-Álvarez, P.J.; Pozo-Bayón, M.A. Analytical Performance of Three Commonly Used Extraction Methods for the Gas Chromatography–Mass Spectrometry Analysis of Wine Volatile Compounds. J. Chromatogr. A 2009, 1216, 7351–7357. [Google Scholar] [CrossRef]
- Jagatić Korenika, A.-M.; Preiner, D.; Tomaz, I.; Jeromel, A. Volatile Profile Characterization of Croatian Commercial Sparkling Wines. Molecules 2020, 25, 4349. [Google Scholar] [CrossRef]
- Lotti, C.; Rubert, J.; Fava, F.; Tuohy, K.; Mattivi, F.; Vrhovsek, U. Development of a Fast and Cost-Effective Gas Chromatography-Mass Spectrometry Method for the Quantification of Short-Chain and Medium-Chain Fatty Acids in Human Biofluids. Anal. Bioanal. Chem. 2017, 409, 5555–5567. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, I.; Ferreira, V. Modulating Fermentative, Varietal and Aging Aromas of Wine Using Non-Saccharomyces Yeasts in a Sequential Inoculation Approach. Microorganisms 2019, 7, 164. [Google Scholar] [CrossRef] [Green Version]
- Versini, G. Sull’aroma Del Vino “Traminer Aromatico” o “Gewürztraminer”. VIGNEVINI 1985, 12, 57–65. [Google Scholar]
- Guth, H. Identification of Character Impact Odorants of Different White Wine Varieties. J. Agric. Food Chem. 1997, 45, 3022–3026. [Google Scholar] [CrossRef]
- Román, T.; Tonidandel, T.; Larcher, R.; Celotti, E.; Nicolini, G. Importance of Polyfunctional Thiols on Semi-Industrial Gewürztraminer Wines and the Correlation to Technological Treatments. Eur. Food Res. Technol. 2018, 244, 379–386. [Google Scholar] [CrossRef]
- Capone, D.L.; Van Leeuwen, K.; Taylor, D.K.; Jeffery, D.W.; Pardon, K.H.; Elsey, G.M.; Sefton, M.A. Evolution and Occurrence of 1,8-Cineole (Eucalyptol) in Australian Wine. J. Agric. Food Chem. 2011, 59, 953–959. [Google Scholar] [CrossRef]
- Fariña, L.; Boido, E.; Carrau, F.; Versini, G.; Dellacassa, E. Terpene Compounds as Possible Precursors of 1,8-Cineole in Red Grapes and Wines. J. Agric. Food Chem. 2005, 53, 1633–1636. [Google Scholar] [CrossRef]
- Mendes-Pinto, M.M. Carotenoid Breakdown Products the—Norisoprenoids—in Wine Aroma. Arch. Biochem. Biophys. 2009, 483, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Sefton, M.A.; Skouroumounis, G.K.; Elsey, G.M.; Taylor, D.K. Occurrence, Sensory Impact, Formation, and Fate of Damascenone in Grapes, Wines, and Other Foods and Beverages. J. Agric. Food Chem. 2011, 59, 9717–9746. [Google Scholar] [CrossRef]
- Versini, G.; Carlin, S.; Dalla Serra, A.; Nicolini, G.; Rapp, A. Formation of 1,1,6-Trimethyl-1,2-Dihydronaphthalene and Other Norisoprenoids in Wine: Considerations on the Kinetics. In Carotenoid-Derived Aroma Compounds; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2001; Volume 802, pp. 285–299. ISBN 0-8412-3729-8. [Google Scholar]
- Winterhalter, P. 1,1,6-Trimethyl-1,2-Dihydronaphthalene (TDN) Formation in Wine. 1. Studies on the Hydrolysis of 2,6,10,10-Tetramethyl-1-Oxaspiro[4.5]Dec-6-Ene-2,8-Diol Rationalizing the Origin of TDN and Related C13 Norisoprenoids in Riesling Wine. Available online: https://pubs.acs.org/doi/abs/10.1021/jf00010a027 (accessed on 19 December 2018).
- Gök, R.; Bechtloff, P.; Ziegler, M.; Schmarr, H.-G.; Fischer, U.; Winterhalter, P. Synthesis of Deuterium-Labeled 1,1,6-Trimethyl-1,2-Dihydronaphthalene (TDN) and Quantitative Determination of TDN and Isomeric Vitispiranes in Riesling Wines by a Stable-Isotope-Dilution Assay. J. Agric. Food Chem. 2019, 67, 6414–6422. [Google Scholar] [CrossRef] [PubMed]
- Carlin, S.; Vrhovsek, U.; Franceschi, P.; Lotti, C.; Bontempo, L.; Camin, F.; Toubiana, D.; Zottele, F.; Toller, G.; Fait, A.; et al. Regional Features of Northern Italian Sparkling Wines, Identified Using Solid-Phase Micro Extraction and Comprehensive Two-Dimensional Gas Chromatography Coupled with Time-of-Flight Mass Spectrometry. Food Chem. 2016, 208, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Zougagh, M.; Ríos, A.; Valcárcel, M. Determination of Total Safranal by in Situ Acid Hydrolysis in Supercritical Fluid Media: Application to the Quality Control of Commercial Saffron. Anal. Chim. Acta 2006, 578, 117–121. [Google Scholar] [CrossRef]
- Winterhalter, P.; Straubinger, M. Saffron-Renewed Interest in an Ancient Spice. Food Rev. Int. 2000, 16, 39–59. [Google Scholar] [CrossRef]
- Carmona, M.; Zalacain, A.; Salinas, M.R.; Alonso, G.L. Generation of Saffron Volatiles by Thermal Carotenoid Degradation. J. Agric. Food Chem. 2006, 54, 6825–6834. [Google Scholar] [CrossRef]
- Acquaviva, V.; D’Auria, M.; Racioppi, R. Changes in Aliphatic Ester Composition of White Wines during Exposition to Light. An HS-SPME-GC-MS Study. J. Wine Res. 2014, 25, 63–74. [Google Scholar] [CrossRef]
- Falcao, L.D.; Lytra, G.; Darriet, P.; Barbe, J.-C. Identification of Ethyl 2-Hydroxy-4-Methylpentanoate in Red Wines, a Compound Involved in Blackberry Aroma. Food Chem. 2012, 132, 230–236. [Google Scholar] [CrossRef]
- Díaz-Maroto, M.C.; Schneider, R.; Baumes, R. Formation Pathways of Ethyl Esters of Branched Short-Chain Fatty Acids during Wine Aging. J. Agric. Food Chem 2005, 53, 3503–3509. [Google Scholar] [CrossRef]
- Riu-Aumatell, M.; Bosch-Fuste, J.; Lopez-Tamames, E.; Buxaderas, S. Development of Volatile Compounds of Cava (Spanish Sparkling Wine) during Long Ageing Time in Contact with Lees. Food Chem. 2006, 95, 237–242. [Google Scholar] [CrossRef]
- Ubeda, C.; Kania-Zelada, I.; del Barrio-Galán, R.; Medel-Marabolí, M.; Gil, M.; Peña-Neira, Á. Study of the Changes in Volatile Compounds, Aroma and Sensory Attributes during the Production Process of Sparkling Wine by Traditional Method. Food Res. Int. 2019, 119, 554–563. [Google Scholar] [CrossRef]
- Grando, M.S.; Versini, G.; Nicolini, G.; Mattivi, F. Selective Use of Wine Yeast Strains Having Different Volatile Phenols Production. Vitis 1993, 32, 43–50. [Google Scholar]
- Waterhouse, A.L.; Sacks, G.L.; Jeffery, D.W. Understanding Wine Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2016; ISBN 978-1-118-62780-8. [Google Scholar]
- Vanbeneden, N.; Saison, D.; Delvaux, F.; Delvaux, F.R. Decrease of 4-Vinylguaiacol during Beer Aging and Formation of Apocynol and Vanillin in Beer. J. Agric. Food Chem. 2008, 56, 11983–11988. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.; Escudero, A.; Fernández, P.; Cacho, J.F. Changes in the Profile of Volatile Compounds in Wines Stored under Oxygen and Their Relationship with the Browning Process. Z. Lebensm. Unters. Forsch. 1997, 205, 392–396. [Google Scholar] [CrossRef]
- Carlin, S.; Masuero, D.; Guella, G.; Vrhovsek, U.; Mattivi, F. Methyl Salicylate Glycosides in Some Italian Varietal Wines. Molecules 2019, 24, 3260. [Google Scholar] [CrossRef] [Green Version]
- Slaghenaufi, D.; Luzzini, G.; Samaniego Solis, J.; Forte, F.; Ugliano, M. Two Sides to One Story—Aroma Chemical and Sensory Signature of Lugana and Verdicchio Wines. Molecules 2021, 26, 2127. [Google Scholar] [CrossRef]
- Rapp, A.; Versini, G.; Ullemeyer, H. 2-Aminoacetophenon: Verursachende Komponente Der, “Untypischen Alterungsnote” (“Naphthalinton”, “Hybridton”) Bei Wein. Vitis 1993, 32, 61–62. [Google Scholar] [CrossRef]
- Perry, D.M.; Hayes, J.E. Effects of Matrix Composition on Detection Threshold Estimates for Methyl Anthranilate and 2-Aminoacetophenone. Foods 2016, 5, 35. [Google Scholar] [CrossRef] [Green Version]
- Christoph, N.; Gessner, M.; Simat, T.J.; Hoenicke, K. Off-Flavor Compounds in Wine and Other Food Products Formed by Enzymatical, Physical, and Chemical Degradation of Tryptophan and Its Metabolites. Adv. Exp. Med. Biol. 1999, 467, 659–669. [Google Scholar] [CrossRef]
- Mattivi, F.; Vrhovšek, U.; Versini, G. Determination of Indole-3-Acetic Acid, Tryptophan and Other Indoles in Must and Wine by High-Performance Liquid Chromatography with Fluorescence Detection. J. Chromatogr. A 1999, 855, 227–235. [Google Scholar] [CrossRef]
- Vrhovsek, U.; Lotti, C.; Masuero, D.; Carlin, S.; Weingart, G.; Mattivi, F. Quantitative Metabolic Profiling of Grape, Apple and Raspberry Volatile Compounds (VOCs) Using a GC/MS/MS Method. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 966, 132–139. [Google Scholar] [CrossRef]







| Quantifier | Qualifier 1 | Calibration Curve | Linearity (μg/L) Split 1:10 (* Split 1:150) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compound | RT | R | W | CAS Number | Q | CE V | q1 | CE V | q1/Q | Equation | R2 | LOQ | Max |
| Isobutyl acetate | 4.527 | × | × | 110-19-0 | 56 > 41 | 9 | 56 > 39 | 21 | 0.32 | y = 0.670244 * x + 4.653750 × 10−6 | 0.996162 | 0.5 | 750 |
| Ethyl butyrate | 4.768 | × | × | 105-54-4 | 71 > 43 | 5 | 116 > 73 | 11 | 0.04 | y = 1.230283 * x + 2.590229 × 10−4 | 0.996698 | 0.1 | 600 |
| Ethyl 2-methylbutyrate | 4.945 | × | × | 7452-79-1 | 102 > 74 | 6 | 102 > 56 | 14 | 0.1 | y = 0.862654 * x + 2.666487 × 10−5 | 0.993428 | 0.05 | 600 |
| Ethyl isovalerate | 5.110 | × | × | 108-64-5 | 88 > 61 | 4 | 85 > 57 | 4 | 1.46 | y = 0.427345 * x + 1.411355 × 10−4 | 0.997103 | 0.15 | 250 |
| Butyl acetate | 5.151 | × | × | 123-86-4 | 56 > 41 | 8 | 56 > 39 | 21 | 0.32 | y = 0.728005 * x + 2.214900 × 10−4 | 0.99692 | 0.5 | 600 |
| Isopentyl acetate | 5.678 | × | 123-92-2 | 70 > 55 | 7 | 55 > 29 | 9 | 0.21 | y = 0.844247 * x + 2.784195 × 10−4 | 0.994801 | 0.5 | 600 * | |
| Ethyl valerate | 5.806 | × | × | 539-82-2 | 85 > 57 | 3 | 101 > 73 | 5 | 0.43 | y = 0.346956 * x + 1.479302 × 10−5 | 0.995213 | 0.075 | 600 |
| 1,8-Cineole | 6.552 | × | × | 470-82-6 | 154 > 84 | 8 | 154 > 69 | 21 | 0.78 | y = 0.156753 * x + 2.082461 × 10−5 | 0.998566 | 0.05 | 380 |
| Ethyl capronate | 6.738 | × | × | 123-66-0 | 88 > 61 | 13 | 101 > 73 | 5 | 1.4 | y = 0.301412 * x + 1.109648 × 10−4 | 0.993592 | 0.5 | 1500 * |
| Hexyl acetate | 7.068 | × | × | 142-92-7 | 56 > 41 | 10 | 61 > 43 | 13 | 0.46 | y = 0.766427 * x + 9.023786 × 10−4 | 0.998808 | 0.5 | 600 |
| Ethyl heptanoate | 7.561 | × | × | 106-30-9 | 113 > 43 | 8 | 113 > 57 | 5 | 0.2 | y = 0.351393 * x + 5.145851 × 10−5 | 0.994433 | 0.1 | 250 |
| cis Rose oxide | 7.752 | × | × | 16409-43-1 | 139 > 69 | 12 | 154 > 112 | 4 | 0.01 | y = 2.264015 * x − 1.932679 × 10−5 | 0.997857 | 0.055 | 364 |
| trans-3-Hexen-1-ol | 7.784 | × | × | 928-97-2 | 82 > 67 | 6 | 82 > 41 | 22 | 0.21 | y = 0.886908 * x − 5.399564 × 10−6 | 0.99977 | 0.075 | 380 |
| trans Rose oxide | 7.879 | × | × | 16409-43-1 | 139 > 69 | 12 | 154 > 139 | 4 | 0.09 | y = 2.327479 * x − 6.575159 × 10−6 | 0.997533 | 0.014 | 163 |
| cis-3-Hexen-1-ol | 7.969 | × | × | 928-96-1 | 82 > 67 | 6 | 82 > 41 | 22 | 0.21 | y = 0.737803 * x + 1.343361 × 10−4 | 0.996857 | 0.1 | 500 |
| Furfurylthiol | 8.311 | × | × | 98-02-2 | 114 > 81 | 5 | 114 > 53 | 23 | 0.15 | y = 0.914263 * x − 0.025057 | 0.995537 | 25 | 600 |
| Ethyl caprylate | 8.321 | × | × | 106-32-1 | 88 > 61 | 8 | 88 > 60 | 8 | 0.64 | y = 0.535577 * x + 3.897772 × 10−4 | 0.996045 | 0.25 | 1500 * |
| Linalool oxide A | 8.431 | × | × | 60047-17-8 | 94 > 79 | 9 | 111 > 93 | 3 | 0.48 | y = 0.880633 * x + 1.053171 × 10−5 | 0.998926 | 0.136 | 326 |
| 1-Heptanol (ISTD) | 8.450 | × | × | 111-70-6 | 70 > 55 | 8 | 70 > 42 | 4 | 0.32 | - | - | - | - |
| Linalool oxide B | 8.624 | × | × | 60047-17-8 | 94 > 79 | 10 | 111 > 93 | 3 | 0.43 | y = 0.901968 * x − 2.790951 × 10−5 | 0.998762 | 0.114 | 274 |
| 2-sec-Butyl-3-methoxypyrazine | 8.804 | × | × | 24168-70-5 | 138 > 123 | 11 | 151 > 83 | 9 | 0.13 | y = 0.572936 * x − 2.320136 × 10−5 | 0.999409 | 0.05 | 380 |
| Benzaldehyde | 9.004 | × | × | 100-52-7 | 105 > 77 | 13 | 106 > 77 | 22 | 0.59 | y = 2.520564 * x + 0.013265 | 0.997263 | 0.1 | 500 |
| Ethyl leucate | 9.021 | × | × | 10348-47-7 | 87 > 69 | 2 | 87 > 41 | 15 | 0.32 | y = 0.738923 * x + 1.965922 × 10−5 | 0.999635 | 0.075 | 500 |
| Linalool | 9.035 | × | × | 126-91-0 | 93 > 77 | 14 | 93 > 91 | 14 | 0.57 | y = 0.712852 * x + 1.982497 × 10−5 | 0.998334 | 0.15 | 500 |
| Terpinen-4-ol | 9.472 | × | × | 20126-76-5 | 93 > 77 | 14 | 136 > 93 | 8 | 0.2 | y = 0.738923 * x + 1.965922 × 10−5 | 0.999635 | 0.075 | 380 |
| Ethyl caprate | 9.607 | × | × | 110-38-3 | 157 > 87 | 11 | 88 > 61 | 4 | 4 | y = 0.110673 * x + 2.153586 × 10−4 | 0.997827 | 0.5 | 75 * |
| Benzylmercaptan | 9.610 | × | × | 100-53-8 | 124 > 91 | 4 | 91 > 65 | 17 | 0.73 | y = 1.697011 * x − 0.007896 | 0.990931 | 2.5 | 125 |
| Phenylacetaldehyde | 9.669 | × | 122-78-1 | 120 > 91 | 12 | 91 > 65 | 16 | 2.06 | y = 0.640671 * x + 7.711668 × 10−4 | 0.999613 | 0.5 | 380 | |
| Safranal | 9.742 | × | × | 116-26-7 | 150 > 121 | 5 | 91 > 65 | 15 | y = 0.424750 * x − 1.216147 × 10−5 | 0.998124 | 0.1 | 500 | |
| Diethyl succinate | 9.809 | × | × | 123-25-1 | 129 > 101 | 4 | 129 > 73 | 14 | 0.3 | y = 1.799192 * x + 8.010092 × 10−4 | 0.999379 | 1 | 5000 * |
| α-Terpineol | 9.954 | × | × | 7785-53-7 | 93 > 77 | 18 | 121 > 91 | 19 | 0.17 | y = 0.883463 * x + 1.341620 × 10−4 | 0.997403 | 0.1 | 500 |
| Valeric acid | 10.099 | × | 109-52-4 | 60 > 42 | 11 | 73 > 55 | 9 | 0.45 | y = 1.063399 * x − 0.006555 | 0.994681 | 5 | 120 | |
| β-Citronellol | 10.223 | × | × | 7540-51-4 | 95 > 67 | 9 | 156 > 95 | 7 | 0.06 | y = 0.234660 * x + 4.673063 × 10−4 | 0.997602 | 0.5 | 500 |
| TDN | 10.236 | × | × | 30364-38-6 | 157 > 142 | 14 | 172 > 157 | 9 | 0.42 | y = 0.318269 * x + 1.753377 × 10−4 | 0.990574 | 1 | 125 |
| Ethyl phenylacetate | 10.371 | × | × | 101-97-3 | 164 > 91 | 6 | 164 > 105 | 3 | 0.15 | y = 1.103282 * x + 1.327135 × 10−5 | 0.999731 | 0.05 | 380 |
| Methyl salicylate | 10.386 | × | × | 119-36-8 | 120 > 92 | 10 | 120 > 64 | 24 | 0.25 | y = 2.769435 * x + 2.479293 × 10−4 | 0.996217 | 0.05 | 500 |
| Nerol | 10.393 | × | × | 106-25-2 | 136 > 121 | 5 | 121 > 105 | 9 | 1.45 | y = 0.030762 * x + 1.555425 × 10−5 | 0.997223 | 1 | 500 |
| Phenylethyl acetate | 10.507 | × | × | 103-45-7 | 104 > 78 | 14 | 91 > 65 | 15 | 0.17 | y = 2.617976 * x + 1.920198 × 10−4 | 0.999673 | 0.05 | 380 |
| β-Damascone | 10.539 | × | × | 23726-91-2 | 177 > 149 | 9 | 123 > 81 | 9 | 2.34 | y = 0.189745 * x + 9.228603 × 10−7 | 0.999728 | 0.25 | 380 |
| β-Damascenone | 10.552 | × | × | 23726-93-4 | 190 > 121 | 5 | 190 > 175 | 6 | 0.7 | y = 0.195451 * x − 3.627043 × 10−6 | 0.999509 | 0.1 | 380 |
| Ethyl laurate | 10.557 | × | 106-33-2 | 101 > 73 | 5 | 88 > 61 | 4 | 0.81 | y = 1.069797 * x + 3.037786 × 10−4 | 0.997895 | 0.15 | 500 | |
| Geraniol | 10.569 | × | × | 106-24-1 | 93 > 77 | 15 | 123 > 81 | 10 | 0.23 | y = 0.112392 * x − 1.567887 × 10−5 | 0.995244 | 0.5 | 500 |
| Guaiacol | 10.675 | × | × | 90-05-1 | 109 > 81 | 10 | 109 > 53 | 21 | 0.18 | y = 1.724700 * x + 7.658686 × 10−4 | 0.995602 | 0.15 | 500 |
| Benzyl alcohol | 10.732 | × | × | 100-51-6 | 108 > 79 | 16 | 108 > 77 | 32 | 0.36 | y = 1.450902 * x + 0.001039 | 0.99974 | 0.1 | 380 |
| trans-Whiskey lactone | 10.799 | × | × | 39212-23-2 | 99 > 71 | 2 | 87 > 69 | 2 | 0.3 | y = 1.236874 * x − 5.675139 × 10−5 | 0.99967 | 0.085 | 216 |
| γ-Octalactone | 10.938 | × | × | 104-50-7 | 85 > 57 | 5 | 100 > 72 | 3 | 0.05 | y = 1.209842 * x − 1.267947 × 10−4 | 0.999779 | 0.25 | 380 |
| β-Ionone | 10.994 | × | × | 79-77-6 | 177 > 162 | 17 | 177 > 147 | 23 | 0.98 | y = 0.643938 * x + 1.506262 × 10−5 | 0.999647 | 0.05 | 380 |
| cis-Whiskey lactone | 11.062 | × | × | 39212-23-2 | 99 > 71 | 2 | 87 > 69 | 2 | 0.5 | y = 1.188140 * x − 6.218725 × 10−5 | 0.996834 | 0.108 | 216 |
| Benzothiazole | 11.110 | × | × | 95-16-9 | 135 > 91 | 17 | 108 > 82 | 20 | 0.66 | y = 0.633120 * x + 3.161581 × 10−4 | 0.996517 | 0.25 | 500 |
| 4-Ethyl guaiacol | 11.253 | × | × | 2785-89-9 | 137 > 94 | 21 | 152 > 137 | 12 | 0.7 | y = 1.417789 * x − 2.338362 × 10−5 | 0.996422 | 0.15 | 500 |
| Octanoic acid | 11.314 | × | × * | 124-07-2 | 60 > 42 | 13 | 73 > 55 | 10 | 0.82 | y = 0.818348 * x + 5.533180 × 10−4 | 0.997182 | 2.5 | 1500 * |
| γ-Nonalactone | 11.328 | × | × | 104-61-0 | 128 > 95 | 6 | 128 | 0.04 | 0.99 | y = 0.077137 * x − 5.774876 × 10−6 | 0.999203 | 0.5 | 380 |
| Ethyl cinnamate | 11.627 | × | × | 103-36-6 | 131 > 77 | 23 | 176 > 131 | 8 | 0.22 | y = 1.777345 * x − 9.711529 × 10−6 | 0.999203 | 0.05 | 380 |
| Nonanoic acid | 11.670 | × | × | 112-05-0 | 60 > 42 | 12 | 129 > 87 | 6 | 0.39 | y = 0.657491 * x − 0.001342 | 0.997586 | 5 | 380 |
| 4-Ethylphenol | 11.706 | × | × | 123-07-9 | 122 > 107 | 11 | 107 > 77 | 16 | 1.76 | y = 2.334519 * x + 4.080627 × 10−5 | 0.99959 | 0.05 | 380 |
| Eugenol | 11.723 | × | × | 97-53-0 | 164 > 149 | 9 | 164 > 104 | 13 | 0.55 | y = 0.657689 * x − 2.787395 × 10−4 | 0.999095 | 0.5 | 380 |
| γ-Decalactone | 11.728 | × | × | 706-14-9 | 128 > 71 | 5 | 128 > 95 | 5 | 0.85 | y = 0.133347 * x − 2.085701 × 10−5 | 0.999513 | 0.25 | 380 |
| 4-Vinylguaiacol | 11.852 | × | × | 7786-61-0 | 135 > 107 | 5 | 150 > 135 | 22 | 0.76 | y = 1.048017 * x − 0.002505 | 0.995304 | 2.5 | 600 |
| δ-Decalactone | 11.936 | × | × | 705-86-2 | 99 > 71 | 4 | 71 > 43 | 6 | 0.33 | y = 1.394024 * x − 2.265714 × 10−4 | 0.990139 | 0.5 | 500 |
| 2-Aminoacetophenone | 11.967 | × | × | 551-93-9 | 135 > 120 | 11 | 135 > 92 | 23 | 0.37 | y = 1.785880 * x + 4.238632 × 10−5 | 0.99684 | 0.05 | 500 |
| Decanoic acid | 12.045 | × | × | 334-48-5 | 73 > 55 | 10 | 129 > 87 | 6 | 0.75 | y = 0.561944 * x − 0.002159 | 0.998304 | 5 | 2500 * |
| Geranic acid | 12.324 | × | × | 459-80-3 | 100 > 82 | 8 | 69 > 39 | 17 | 1.06 | y = 0.140501 * x − 0.001569 | 0.997411 | 10 | 380 |
| Menthalactone | 12.448 | × | × | 13341-72-5 | 166 > 81 | 14 | 166 > 110 | 5 | 0.45 | y = 0.555579 * x − 1.564872 × 10−5 | 0.997422 | 0.1 | 500 * |
| γ-Dodecalactone | 12.624 | × | × | 2305-05-7 | 85 > 57 | 4 | 69 > 41 | 10 | 0.36 | y = 0.930277 * x − 7.157235 × 10−4 | 0.997274 | 1 | 500 |
| Zingerone | 14.776 | × | × | 122-48-5 | 194 > 137 | 15 | 194 > 151 | 9 | 0.23 | y = 0.854252 * x + 4.546328 × 10−5 | 0.99662 | 0.05 | 500 |
| Time Point | t0 | t1 | t2 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample Code Compounds | G1 | G2 | G3 | G4 | G5 | G6 | G7 | G1 | G2 | G3 | G4 | G5 | G6 | G7 | G1 | G2 | G3 | G4 | G5 | G6 | G7 |
| isobutyl acetate | 49.70 | 43.09 | 60.40 | 53.73 | 36.66 | 31.96 | 47.51 | 24.80 | 39.33 | 33.14 | 39.85 | 23.61 | 27.09 | 28.00 | 18.51 | 34.82 | 20.65 | 35.64 | 19.11 | 21.34 | 23.87 |
| ethyl butyrate | 407.86 | 376.50 | 385.85 | 282.44 | 390.77 | 379.65 | 532.53 | 407.64 | 427.78 | 386.98 | 317.20 | 394.80 | 429.93 | 452.43 | 408.11 | 435.94 | 348.10 | 312.81 | 393.66 | 394.32 | 450.39 |
| ethyl 2-methylbutyrate | 16.79 | 7.07 | 5.86 | 16.38 | 8.34 | 6.03 | 7.90 | 33.90 | 17.56 | 12.66 | 35.09 | 18.71 | 14.81 | 15.82 | 43.84 | 24.17 | 13.54 | 39.93 | 24.37 | 16.82 | 20.70 |
| ethyl isovalerate | 35.87 | 14.18 | 9.80 | 31.92 | 16.59 | 11.64 | 14.08 | 72.26 | 37.94 | 23.48 | 72.40 | 38.51 | 30.89 | 30.49 | 92.76 | 52.22 | 25.67 | 81.72 | 49.95 | 35.56 | 40.10 |
| butyl acetate | 2.29 | 1.71 | 6.29 | 2.11 | 2.04 | 1.74 | 1.71 | 0.744 | 1.27 | 3.02 | 1.17 | 1.03 | 1.28 | 0.808 | 0.427 | 1.00 | 1.54 | 0.728 | 0.631 | 0.884 | 0.528 |
| ethyl valerate | 1.88 | 1.55 | 1.35 | 2.18 | 1.21 | 2.04 | 1.25 | 2.00 | 1.81 | 1.48 | 2.39 | 1.46 | 2.23 | 1.27 | 2.14 | 1.94 | 1.39 | 2.24 | 1.41 | 1.94 | 1.45 |
| 1,8-cineole | 0.071 | n.d. | n.d. | n.d. | n.d. | n.d. | 0.066 | 3.65 | 1.14 | 1.32 | 2.68 | 2.14 | 1.02 | 3.47 | 6.44 | 2.36 | 1.84 | 3.88 | 3.67 | 1.80 | 5.00 |
| ethyl capronate (*) | 887.12 | 627.37 | 839.23 | 565.56 | 759.55 | 770.59 | 1017 | 641.15 | 582.64 | 631.28 | 449.45 | 641.25 | 662.52 | 773.39 | 683.74 | 558.69 | 638.59 | 366.36 | 611.46 | 506.69 | 733.75 |
| hexyl acetate | 121.64 | 50.58 | 152.05 | 51.17 | 48.99 | 29.10 | 47.29 | 34.10 | 28.12 | 51.32 | 17.22 | 21.14 | 14.08 | 18.13 | 15.99 | 18.05 | 30.29 | 6.96 | 9.68 | 7.46 | 10.19 |
| ethyl heptanoate | 0.943 | 1.41 | 1.02 | 1.34 | 0.970 | 1.84 | 0.907 | 0.574 | 1.06 | 0.610 | 0.906 | 0.800 | 1.28 | 0.687 | 0.659 | 1.02 | 0.579 | 0.666 | 0.689 | 0.955 | 0.680 |
| cis rose oxide | 3.77 | 4.41 | 2.84 | 2.91 | 3.57 | 5.24 | 6.69 | 2.58 | 3.89 | 2.06 | 2.15 | 2.89 | 4.45 | 5.61 | 2.53 | 3.46 | 1.72 | 1.64 | 2.55 | 3.41 | 4.78 |
| trans-3-hexen-1-ol | 145.83 | 47.11 | 74.97 | 54.99 | 79.77 | 53.95 | 57.23 | 123.30 | 41.20 | 70.10 | 49.37 | 70.73 | 46.47 | 68.84 | 120.10 | 39.03 | 64.80 | 46.70 | 67.11 | 44.62 | 64.78 |
| trans rose oxide | 0.380 | 0.667 | 0.382 | 0.312 | 0.448 | 0.922 | 1.08 | 0.251 | 0.366 | 0.218 | 0.218 | 0.285 | 0.416 | 0.505 | 0.246 | 0.331 | 0.184 | 0.175 | 0.251 | 0.323 | 0.427 |
| cis-3-hexen-1-ol | 51.65 | 15.36 | 68.75 | 20.46 | 26.57 | 25.26 | 29.01 | 43.72 | 13.49 | 62.42 | 18.30 | 24.17 | 21.71 | 33.97 | 43.23 | 13.26 | 60.82 | 17.64 | 23.05 | 20.83 | 32.24 |
| ethyl caprylate (*) | 1053 | 625.76 | 958.33 | 692.90 | 771.87 | 754.53 | 894.97 | 406.24 | 279.62 | 362.73 | 288.33 | 339.27 | 321.48 | 428.00 | 436.36 | 264.66 | 313.58 | 189.89 | 305.28 | 242.75 | 388.49 |
| linalool oxide A | 24.21 | 17.10 | 12.75 | 19.43 | 20.72 | 12.81 | 14.32 | 217.02 | 150.52 | 134.68 | 210.77 | 177.96 | 127.06 | 185.41 | 301.42 | 217.81 | 159.02 | 269.25 | 233.78 | 173.44 | 246.64 |
| linalool oxide B | 11.64 | 8.88 | 6.52 | 9.37 | 10.48 | 7.67 | 7.66 | 129.58 | 88.83 | 81.72 | 126.58 | 106.32 | 75.85 | 109.63 | 179.60 | 128.97 | 98.75 | 159.84 | 137.60 | 101.91 | 143.17 |
| benzaldehyde | 2.10 | 3.09 | 1.34 | 4.07 | 7.72 | 4.23 | 3.15 | 8.07 | 5.56 | 3.89 | 12.13 | 14.88 | 7.94 | 5.79 | 7.92 | 7.69 | 6.17 | 13.79 | 19.88 | 11.78 | 9.18 |
| linalool | 367.71 | 140.12 | 123.91 | 154.15 | 196.29 | 143.23 | 378.40 | 66.89 | 125.00 | 38.17 | 52.25 | 66.52 | 133.71 | 110.20 | 26.28 | 78.64 | 17.74 | 25.01 | 32.25 | 86.46 | 45.77 |
| ethyl leucate | 75.14 | 45.39 | 33.62 | 70.09 | 50.66 | 38.91 | 42.97 | 121.27 | 79.79 | 59.10 | 116.44 | 84.57 | 65.81 | 89.19 | 137.10 | 95.40 | 59.69 | 127.84 | 97.19 | 76.71 | 105.88 |
| terpinen-4-ol | 2.81 | 2.23 | 2.05 | 3.27 | 3.13 | 1.70 | 2.12 | 8.62 | 9.52 | 5.21 | 8.32 | 9.46 | 7.20 | 9.67 | 7.59 | 9.30 | 4.04 | 6.77 | 8.21 | 6.94 | 8.96 |
| ethyl caprate | 207.84 | 93.60 | 240.73 | 146.40 | 169.24 | 112.94 | 233.19 | 45.03 | 16.80 | 53.46 | 31.49 | 32.39 | 22.12 | 44.01 | 43.86 | 17.10 | 27.24 | 14.82 | 27.04 | 15.16 | 34.18 |
| phenylacetaldehyde | 25.76 | 14.96 | 12.34 | 24.88 | 20.06 | 21.90 | 17.47 | 23.50 | 12.76 | 16.89 | 17.86 | 16.23 | 16.89 | 14.90 | 41.03 | 15.00 | 51.91 | 30.86 | 23.16 | 38.91 | 25.80 |
| safranal | 0.161 | 0.128 | 0.141 | 0.144 | 0.145 | 0.161 | 0.127 | 1.08 | 0.732 | 0.828 | 1.05 | 0.896 | 0.836 | 0.800 | 1.46 | 1.04 | 0.949 | 1.15 | 1.16 | 1.14 | 1.04 |
| diethyl succinate (*) | 3083 | 3991 | 2078 | 3709 | 3672 | 6924 | 1753 | 8086 | 6315 | 4246 | 7754 | 6674 | 9038 | 5329 | 11127 | 8328 | 5929 | 9998 | 8686 | 11045 | 7955 |
| α-terpineol | 138.07 | 64.98 | 55.31 | 73.08 | 90.92 | 64.31 | 144.29 | 397.02 | 298.50 | 190.75 | 283.36 | 305.72 | 291.85 | 472.38 | 323.73 | 330.57 | 156.21 | 236.26 | 273.95 | 314.13 | 425.30 |
| valeric acid | 25.93 | 25.57 | 26.47 | 29.89 | 20.11 | 26.04 | 17.06 | 24.64 | 23.45 | 24.11 | 28.51 | 22.98 | 26.58 | 20.15 | 22.60 | 21.54 | 26.44 | 25.39 | 18.61 | 24.54 | 18.75 |
| α-citronellol | 43.44 | 97.69 | 30.66 | 45.07 | 63.06 | 107.05 | 98.73 | 10.61 | 43.45 | 10.73 | 9.66 | 18.97 | 51.22 | 33.81 | 6.03 | 26.47 | 8.87 | 5.59 | 10.72 | 31.95 | 18.68 |
| TDN | 1.37 | 0.696 | 0.808 | 1.11 | 0.990 | 0.589 | 0.553 | 16.88 | 7.35 | 17.78 | 17.20 | 15.97 | 8.13 | 9.05 | 21.15 | 11.08 | 13.30 | 11.02 | 17.60 | 9.53 | 9.75 |
| ethyl phenylacetate | 6.84 | 6.49 | 6.41 | 9.51 | 6.93 | 10.54 | 5.17 | 11.81 | 12.21 | 12.69 | 17.24 | 13.20 | 19.21 | 9.61 | 14.46 | 15.50 | 13.73 | 19.42 | 16.11 | 23.41 | 11.95 |
| methyl salicylate | 0.948 | 1.05 | 0.676 | 0.992 | 1.42 | 0.918 | 2.07 | 1.81 | 1.87 | 1.09 | 2.03 | 2.77 | 1.13 | 2.34 | 1.77 | 1.92 | 1.06 | 1.75 | 2.33 | 1.15 | 2.18 |
| nerol | 34.74 | 50.15 | 23.09 | 21.49 | 46.49 | 84.22 | 206.48 | 3.57 | 10.58 | n.d | n.d | 3.42 | 9.43 | 14.25 | n.d | 5.29 | n.d | n.d | n.d | 5.36 | n.d |
| phenylethyl acetate | 309.49 | 180.43 | 295.92 | 281.18 | 154.16 | 214.30 | 115.60 | 134.92 | 111.58 | 151.80 | 125.26 | 80.07 | 133.92 | 61.08 | 83.46 | 85.24 | 100.26 | 84.47 | 56.03 | 106.09 | 45.46 |
| β-damascenone | 2.11 | 1.86 | 2.57 | 3.35 | 1.78 | 2.43 | 2.65 | 4.31 | 2.20 | 3.93 | 4.46 | 2.75 | 2.87 | 2.76 | 4.64 | 2.27 | 3.89 | 4.36 | 3.06 | 3.45 | 3.24 |
| geraniol | 192.97 | 97.42 | 51.66 | 84.00 | 114.83 | 160.03 | 462.34 | 14.90 | 33.26 | 8.73 | 14.38 | 15.62 | 31.95 | 23.99 | 5.87 | 20.77 | 6.03 | 6.63 | 7.46 | 21.38 | 10.44 |
| guaiacol | 0.668 | 0.937 | 0.732 | 2.00 | 0.706 | 0.832 | 0.587 | 1.51 | 1.97 | 1.57 | 3.24 | 1.96 | 1.99 | 1.71 | 2.14 | 2.76 | 2.70 | 3.85 | 2.75 | 2.81 | n.d |
| benzyl alcohol | 190.26 | 147.24 | 91.30 | 78.59 | 100.32 | 173.85 | 109.19 | 159.93 | 122.57 | 82.55 | 71.09 | 90.25 | 149.30 | 121.54 | 163.21 | 123.39 | 106.34 | 70.65 | 89.08 | 148.97 | 117.21 |
| trans-whiskey lactone | n.d | 0.256 | 0.665 | 1.05 | 0.402 | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d |
| γ-octalactone | 0.969 | 2.22 | 1.43 | 2.05 | 1.11 | 2.97 | 1.03 | 4.94 | 3.00 | 2.62 | 4.94 | 2.97 | 5.83 | 3.41 | n.d | 1.49 | 1.16 | 1.12 | 1.11 | 3.12 | 1.00 |
| cis-whiskey lactone | n.d | 0.322 | 1.03 | 1.72 | 0.719 | 0.478 | n.d | n.d | n.d | 1.02 | 1.23 | n.d | n.d | n.d | n.d | 0.634 | 0.831 | 1.86 | 0.678 | 0.618 | 8.39 |
| benzothiazole | 0.305 | 0.114 | 0.439 | 0.090 | 0.141 | 0.424 | n.d | 0.841 | 0.683 | 0.873 | 0.567 | 0.773 | 0.795 | 0.506 | 0.855 | 0.707 | 1.68 | 0.709 | 0.669 | 0.874 | 0.490 |
| 4-ethyl guaiacol | 0.217 | 0.195 | 0.149 | 0.541 | 0.384 | 0.253 | 0.251 | 0.289 | 0.206 | 0.227 | 0.584 | 0.317 | 0.232 | 0.239 | 0.397 | 0.276 | 0.373 | 0.673 | 0.381 | 0.319 | 0.279 |
| γ-nonalactone | 4.60 | 11.05 | 7.56 | 7.74 | 6.54 | 17.60 | 6.46 | 4.57 | 10.34 | 7.37 | 7.85 | 6.50 | 16.35 | 5.03 | 4.88 | 11.81 | 13.19 | 8.72 | 7.00 | 17.96 | 5.91 |
| ethyl cinnamate | 0.990 | 0.783 | 1.39 | 0.841 | 0.706 | 0.966 | 0.596 | 0.765 | 0.485 | 0.979 | 0.683 | 0.714 | 0.762 | 0.372 | 0.791 | 0.619 | 0.945 | 0.613 | 0.570 | 0.789 | 0.442 |
| nonanoic acid | 71.10 | 44.38 | 36.35 | 58.65 | 54.77 | 47.11 | 33.04 | 108.32 | 83.46 | 68.51 | 100.68 | 96.69 | 81.26 | 75.45 | 107.48 | 89.85 | 87.52 | 98.33 | 101.69 | 86.67 | 80.57 |
| γ-decalactone | 1.29 | 1.73 | 1.45 | 1.57 | 0.966 | 2.35 | 2.04 | 1.21 | 1.86 | 1.58 | 1.55 | 1.13 | 2.25 | 1.94 | 1.28 | 1.75 | 1.52 | 1.52 | 1.09 | 2.09 | 1.90 |
| 4-ethyl-phenol | 0.200 | 0.290 | 0.525 | 0.874 | 0.381 | 0.246 | 0.249 | 0.649 | 0.604 | 0.653 | 1.15 | 0.658 | 0.471 | 0.312 | 0.808 | 0.441 | 0.895 | 1.23 | 0.623 | 0.406 | 0.383 |
| eugenol | 5.55 | 4.13 | 5.26 | 3.66 | 4.89 | 5.48 | 7.98 | 6.88 | 4.63 | 6.19 | 4.78 | 5.68 | 6.15 | 8.20 | 7.01 | 4.86 | 5.73 | 4.76 | 5.83 | 6.17 | 8.47 |
| 4-vinylguaiacol | 876.34 | 652.95 | 313.96 | 865.53 | 653.64 | 992.86 | 546.38 | 280.06 | 387.24 | 330.91 | 271.41 | 247.19 | 384.19 | 405.91 | 265.65 | 433.23 | 312.17 | 228.34 | 240.37 | 303.36 | 372.68 |
| δ-decalactone | 9.88 | 6.72 | 6.06 | 6.61 | 8.62 | 10.48 | 7.30 | 23.03 | 15.33 | 13.41 | 16.70 | 20.36 | 21.18 | 20.58 | 22.53 | 14.47 | 11.62 | 15.22 | 20.69 | 20.26 | 19.72 |
| 2-aminoacetophenone | 0.173 | 0.268 | 0.270 | 0.192 | 0.225 | 0.271 | 0.222 | 0.324 | 0.500 | 0.350 | 0.453 | 0.663 | 0.306 | 0.416 | 0.340 | 0.805 | 0.433 | 0.483 | 0.927 | 0.406 | 0.934 |
| decanoic acid (*) | 2365 | 1201 | 3015 | 1363 | 1870 | 1280 | 2759 | 2485 | 1390 | 3635 | 1420 | 2138 | 1403 | 3016 | 2237 | 1297 | 3076 | 1218 | 1937 | 1339 | 2650 |
| geranic acid (*) | 333.07 | 361.97 | 208.37 | 228.28 | 336.24 | 377.52 | 623.64 | 472.68 | 488.59 | 292.25 | 397.00 | 427.23 | 521.25 | 561.39 | 389.52 | 453.40 | 230.24 | 346.42 | 373.55 | 507.17 | 479.70 |
| γ-dodecalactone | 46.45 | 39.74 | 19.52 | 19.94 | 57.89 | 135.39 | 44.56 | 29.38 | 23.14 | 14.16 | 15.82 | 39.66 | 111.84 | 29.49 | 32.47 | 25.06 | 49.70 | 13.17 | 35.73 | 81.24 | 24.15 |
| zingerone | 23.12 | 33.59 | 17.51 | 18.07 | 24.06 | 31.76 | 22.51 | 27.50 | 36.77 | 19.60 | 22.02 | 26.92 | 34.25 | 28.89 | 28.58 | 37.56 | 18.03 | 21.26 | 26.87 | 32.78 | 29.48 |
| Time Point | t0 | t1 | t2 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample Code Compounds | T1 | T2 | T3 | T4 | T5 | T6 | T7 | T1 | T2 | T3 | T4 | T5 | T6 | T7 | T1 | T2 | T3 | T4 | T5 | T6 | T7 |
| isobutyl acetate | 57.41 | 59.48 | 55.33 | 58.84 | 51.22 | 58.79 | 58.57 | 46.21 | 53.39 | 50.93 | 56.65 | 64.27 | 64.95 | 58.69 | 48.81 | 52.45 | 50.68 | 58.28 | 71.77 | 64.35 | 60.14 |
| ethyl butyrate | 168.24 | 257.81 | 155.78 | 161.86 | 199.52 | 141.15 | 191.17 | 148.64 | 267.62 | 163.87 | 160.39 | 223.13 | 171.94 | 202.27 | 162.90 | 275.77 | 170.41 | 165.86 | 235.84 | 176.21 | 210.07 |
| ethyl 2-methylbutyrate | 10.57 | 6.15 | 23.96 | 12.11 | 12.81 | 15.78 | 12.51 | 16.10 | 11.52 | 37.60 | 19.91 | 23.60 | 31.60 | 21.10 | 21.75 | 14.77 | 47.16 | 26.57 | 31.53 | 41.16 | 26.99 |
| ethyl isovalerate | 20.69 | 12.82 | 33.38 | 21.43 | 18.53 | 27.15 | 17.66 | 31.03 | 25.58 | 51.80 | 36.44 | 36.22 | 56.22 | 30.64 | 41.49 | 32.42 | 64.85 | 48.39 | 48.34 | 73.11 | 39.59 |
| butyl acetate | 0.812 | 1.87 | 1.17 | 1.34 | 1.53 | 1.38 | 1.89 | 0.446 | 1.37 | 1.03 | 1.13 | 1.88 | 1.43 | 1.60 | 0.399 | 1.34 | 1.11 | 1.11 | 2.08 | 1.41 | 1.59 |
| isopentyl acetate (*) | 1134 | 1222 | 1045 | 851.92 | 534.52 | 966.93 | 764.53 | 583.50 | 754.77 | 619.80 | 559.72 | 477.84 | 716.72 | 529.70 | 545.52 | 630.17 | 511.77 | 475.04 | 477.22 | 585.28 | 460.93 |
| ethyl valerate | 0.779 | 2.05 | 0.939 | 0.872 | 1.36 | 0.662 | 3.19 | 0.625 | 1.97 | 0.957 | 0.869 | 1.37 | 0.765 | 3.11 | 0.738 | 2.07 | 1.06 | 0.961 | 1.42 | 0.862 | 3.27 |
| 1,8-cineole | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d | 0.067 | 0.079 | n.d | n.d | n.d | 0.064 | 0.087 | 0.104 | 0.119 | 0.071 | 0.068 | 0.080 | 0.102 |
| ethyl capronate | 308.90 | 341.96 | 228.02 | 260.40 | 199.22 | 228.13 | 245.68 | 186.99 | 243.66 | 168.32 | 184.84 | 155.52 | 213.41 | 191.81 | 210.96 | 230.38 | 173.81 | 193.49 | 154.97 | 217.36 | 190.41 |
| hexyl acetate | 18.18 | 15.93 | 14.99 | 9.24 | 1.90 | 7.12 | 5.87 | 10.71 | 12.54 | 7.90 | 4.51 | 1.97 | 5.58 | 5.49 | 6.34 | 5.90 | 5.09 | 3.43 | 1.67 | 3.42 | 3.03 |
| ethyl heptanoate | 0.847 | 0.949 | 0.702 | 0.539 | 1.04 | 0.407 | 1.15 | 0.400 | 0.523 | 0.411 | 0.299 | 0.604 | 0.323 | 0.699 | 0.449 | 0.448 | 0.438 | 0.311 | 0.600 | 0.348 | 0.661 |
| trans-3-hexen-1-ol | 51.82 | 36.95 | 23.29 | 46.12 | 25.49 | 49.66 | 21.53 | 43.11 | 32.78 | 21.42 | 41.74 | 23.86 | 48.54 | 20.48 | 43.33 | 31.92 | 20.94 | 41.87 | 23.00 | 44.73 | 20.12 |
| cis-3-hexen-1-ol | 217.23 | 189.21 | 102.48 | 160.01 | 92.86 | 173.96 | 83.17 | 175.85 | 167.27 | 93.11 | 142.44 | 86.85 | 167.20 | 77.59 | 177.19 | 163.65 | 91.52 | 143.41 | 85.29 | 157.79 | 77.62 |
| ethyl caprylate | 323.11 | 349.92 | 311.94 | 269.26 | 184.95 | 198.17 | 234.68 | 97.42 | 101.24 | 106.61 | 79.33 | 65.28 | 91.43 | 87.01 | 95.62 | 84.14 | 104.01 | 83.78 | 60.55 | 90.15 | 78.39 |
| linalool oxide A | 3.14 | 1.72 | 3.62 | 2.49 | 3.99 | 10.71 | 3.59 | 11.27 | 7.45 | 9.58 | 8.91 | 11.59 | 21.67 | 11.66 | 13.91 | 9.21 | 12.40 | 12.19 | 14.78 | 24.80 | 15.23 |
| linalool oxide B | 1.61 | 1.09 | 1.81 | 1.37 | 2.35 | 5.94 | 2.14 | 6.39 | 4.53 | 5.25 | 5.15 | 6.84 | 12.41 | 6.91 | 7.93 | 5.54 | 6.83 | 7.06 | 8.65 | 14.15 | 8.88 |
| benzaldehyde | 3.72 | 13.08 | 25.88 | 5.05 | 17.20 | 2.93 | 12.71 | 4.28 | 13.80 | 36.00 | 4.89 | 16.72 | 2.75 | 17.15 | 5.94 | 12.89 | 36.19 | 4.95 | 15.82 | 3.46 | 14.91 |
| linalool | 12.19 | 13.96 | 12.68 | 9.72 | 12.27 | 10.54 | 11.82 | 4.22 | 5.62 | 3.31 | 3.79 | 7.18 | 3.35 | 5.17 | 3.02 | 3.71 | 2.05 | 2.26 | 4.34 | 1.97 | 3.21 |
| ethyl leucate | 157.61 | 70.26 | 92.70 | 86.28 | 86.70 | 101.36 | 103.64 | 174.37 | 82.24 | 119.71 | 114.53 | 121.85 | 138.63 | 131.78 | 183.80 | 83.39 | 127.10 | 121.97 | 134.37 | 144.32 | 140.05 |
| terpinen-4-ol | 0.547 | 1.96 | 0.280 | 0.426 | 0.413 | 0.352 | 0.678 | 0.589 | 1.62 | 0.363 | 0.386 | 0.482 | 0.341 | 0.688 | 0.582 | 1.31 | 0.353 | 0.388 | 0.473 | 0.338 | 0.598 |
| ethyl caprate | 65.81 | 64.87 | 117.89 | 67.48 | 30.91 | 25.45 | 27.53 | 6.42 | 5.10 | 14.91 | 6.52 | 3.72 | 3.92 | 3.84 | 6.24 | 3.56 | 10.53 | 6.84 | 3.36 | 3.95 | 2.42 |
| safranal | 0.141 | 0.148 | 0.150 | 0.137 | 0.121 | 0.111 | 0.143 | 0.807 | 0.781 | 0.737 | 0.640 | 0.825 | 0.745 | 0.865 | 1.04 | 0.926 | 0.973 | 0.862 | 1.11 | 0.928 | 1.12 |
| α-terpineol | 4.63 | 5.32 | 7.27 | 4.06 | 3.65 | 5.10 | 4.05 | 12.45 | 14.52 | 12.44 | 10.49 | 12.84 | 11.24 | 12.25 | 12.49 | 14.24 | 11.95 | 10.42 | 14.42 | 10.61 | 12.76 |
| β-citronellol | 21.84 | 23.93 | 12.16 | 14.26 | 18.56 | 13.17 | 12.92 | 8.93 | 12.97 | 6.36 | 8.88 | 11.06 | 6.62 | 6.38 | 6.64 | 6.43 | 2.65 | 3.81 | 7.39 | 2.80 | 4.95 |
| TDN | 0.399 | 0.267 | 0.704 | 0.262 | n.d | n.d | 0.477 | 5.25 | 2.73 | 4.93 | 2.72 | 3.40 | 3.73 | 7.85 | 5.75 | 3.58 | 5.95 | 4.62 | 5.86 | 6.20 | 8.91 |
| ethyl phenylacetate | 9.47 | 5.61 | 12.23 | 6.48 | 9.36 | 9.21 | 12.33 | 13.24 | 8.54 | 17.11 | 9.85 | 13.91 | 13.71 | 18.48 | 15.82 | 9.85 | 20.01 | 12.01 | 16.93 | 16.24 | 22.41 |
| methyl salicylate | 2.73 | 1.72 | 1.99 | 2.24 | 6.25 | 4.70 | 3.13 | 2.74 | 1.72 | 2.27 | 2.68 | 6.38 | 4.88 | 3.56 | 2.72 | 1.67 | 2.26 | 2.71 | 6.41 | 4.82 | 3.43 |
| nerol | 7.63 | 3.99 | 2.59 | 2.43 | 9.77 | 4.00 | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d |
| phenylethyl acetate | 162.60 | 97.59 | 205.32 | 74.60 | 74.12 | 134.05 | 90.11 | 98.89 | 65.08 | 136.79 | 56.08 | 74.53 | 94.64 | 67.30 | 87.60 | 55.74 | 113.51 | 50.94 | 79.06 | 81.61 | 61.16 |
| β-damascenone | 2.61 | 2.84 | 2.90 | 2.00 | 2.55 | 2.00 | 1.95 | 2.98 | 3.94 | 4.57 | 2.98 | 4.32 | 2.88 | 2.95 | 3.59 | 3.76 | 4.44 | 3.04 | 4.53 | 3.68 | 2.67 |
| ethyl laurate | 2.25 | 1.55 | 1.85 | 1.42 | 1.26 | 0.634 | 0.597 | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d |
| geraniol | 6.59 | 7.00 | 3.63 | 4.71 | 10.99 | 5.85 | 9.38 | 1.57 | 2.00 | 1.35 | 1.50 | 3.11 | 1.23 | 1.97 | 1.19 | 1.40 | n.d | 1.06 | 1.72 | n.d | 1.31 |
| guaiacol | 2.49 | 4.44 | 4.79 | 3.54 | 6.78 | 5.14 | 9.28 | 12.78 | 20.61 | 16.16 | 18.42 | 28.24 | 18.89 | 23.28 | 16.00 | 25.04 | 21.09 | 25.02 | 37.36 | 23.93 | 28.78 |
| benzyl alcohol | 158.67 | 145.93 | 453.65 | 148.69 | 166.99 | 283.95 | 174.16 | 139.25 | 139.53 | 422.92 | 143.42 | 165.20 | 275.95 | 169.38 | 149.14 | 140.61 | 435.27 | 151.65 | 175.04 | 283.55 | 178.19 |
| trans-whiskey lactone | 3.99 | 0.453 | 0.397 | 1.22 | 8.39 | 7.80 | 23.91 | 3.69 | 0.345 | 0.387 | 1.27 | 8.44 | 7.82 | 23.56 | 3.75 | 0.349 | 0.406 | 1.28 | 8.88 | 8.18 | 24.25 |
| γ-octalactone | 0.694 | 0.801 | 0.613 | 0.546 | 0.853 | 0.778 | 1.04 | 1.02 | 0.885 | 0.802 | 0.652 | 1.43 | 1.12 | 1.08 | 0.929 | 0.709 | 0.918 | 0.724 | 1.25 | 0.983 | 1.23 |
| β-ionone | 0.123 | 0.228 | 0.101 | 0.112 | 0.100 | 0.133 | 0.088 | 0.097 | 0.139 | 0.088 | 0.104 | 0.096 | 0.119 | 0.051 | 0.096 | 0.101 | 0.073 | 0.085 | 0.090 | 0.113 | n.d |
| cis-whiskey lactone | 3.17 | 0.910 | 1.08 | 2.81 | 21.37 | 12.83 | 57.68 | 2.96 | 0.704 | 0.999 | 3.07 | 21.63 | 12.77 | 56.68 | 3.13 | 0.645 | 0.945 | 3.27 | 22.13 | 12.72 | 58.45 |
| benzothiazole | 0.231 | 0.094 | 0.739 | n.d | 1.23 | n.d | 1.03 | 0.650 | 0.609 | 1.21 | 0.550 | 1.82 | 0.501 | 1.42 | 0.690 | 0.460 | 1.12 | 0.463 | 1.78 | 0.494 | 1.38 |
| 4-ethyl guaiacol | 0.562 | 2.21 | 0.438 | 0.970 | 0.662 | 1.58 | 10.52 | 0.594 | 2.20 | 0.495 | 1.07 | 0.751 | 1.64 | 10.99 | 0.598 | 2.24 | 0.536 | 1.10 | 0.782 | 1.67 | 11.26 |
| γ-nonalactone | 6.27 | 9.04 | 5.56 | 4.79 | 13.65 | 6.75 | 13.34 | 6.60 | 9.68 | 6.57 | 5.97 | 15.71 | 7.78 | 14.93 | 7.01 | 10.76 | 7.14 | 6.41 | 16.91 | 8.16 | 15.96 |
| octanoic acid (*) | 3103 | 2737 | 2333 | 2330 | 1623 | 2030 | 2027 | 3244 | 3268 | 2779 | 2751 | 1988 | 2384 | 2467 | 3110 | 2976 | 2420 | 2458 | 1808 | 2173 | 2183 |
| ethyl cinnamate | 0.543 | 1.11 | 1.03 | 0.444 | 1.91 | 0.647 | 0.921 | 0.383 | 0.994 | 0.766 | 0.345 | 1.56 | 0.514 | 0.488 | 0.440 | 0.813 | 0.858 | 0.333 | 1.57 | 0.511 | 0.871 |
| nonanoic acid | 72.39 | 73.51 | 107.82 | 87.43 | 69.62 | 102.95 | 84.94 | 94.79 | 80.77 | 118.66 | 113.05 | 90.22 | 120.94 | 98.51 | 90.90 | 83.22 | 115.94 | 108.99 | 93.67 | 114.98 | 101.40 |
| γ-decalactone | 0.951 | 1.00 | 0.859 | 0.277 | 0.979 | 0.494 | 0.962 | 0.89 | 0.79 | 0.72 | 0.40 | 1.04 | 0.490 | 0.831 | 0.926 | 0.747 | 0.683 | 0.383 | 1.05 | 0.436 | 0.885 |
| 4-ethyl-phenol | 8.01 | 12.79 | 1.96 | 7.71 | 1.41 | 26.11 | 192.31 | 8.34 | 13.56 | 2.08 | 8.64 | 1.64 | 28.37 | 207.30 | 8.38 | 12.84 | 1.99 | 8.38 | 1.63 | 27.28 | 199.22 |
| eugenol | 3.67 | 4.18 | 2.75 | 2.92 | 8.62 | 7.21 | 10.32 | 3.95 | 4.75 | 3.38 | 3.40 | 9.48 | 7.72 | 11.31 | 4.19 | 4.83 | 3.47 | 3.46 | 9.62 | 7.69 | 11.59 |
| 4-vinylguaiacol | 6.69 | 7.89 | 7.78 | 5.23 | 5.17 | 7.05 | 11.32 | 14.36 | 16.38 | 11.73 | 9.55 | 11.10 | 11.39 | 15.89 | 17.13 | 20.12 | 16.54 | 13.34 | 16.43 | 15.97 | 20.55 |
| δ-decalactone | 5.00 | 7.19 | 7.07 | 5.80 | 7.84 | 6.92 | 7.72 | 6.04 | 6.97 | 8.74 | 7.35 | 10.16 | 7.52 | 11.71 | 7.43 | 7.38 | 9.20 | 7.87 | 11.47 | 10.97 | 12.56 |
| 2-aminoacetophenone | 0.226 | 0.408 | 0.243 | 0.257 | 0.228 | 0.254 | 0.266 | 0.208 | 0.236 | 0.210 | 0.220 | 0.216 | 0.198 | 0.235 | 0.164 | 0.208 | 0.198 | 0.175 | 0.155 | 0.172 | 0.236 |
| decanoic acid (*) | 946.90 | 629.04 | 1137 | 944.74 | 444.79 | 438.11 | 371.66 | 841.86 | 643.68 | 1227 | 899.36 | 461.66 | 440.14 | 396.50 | 820.80 | 610.97 | 1078 | 829.52 | 431.54 | 417.26 | 347.55 |
| geranic acid | 37.38 | 38.16 | 30.08 | 33.64 | 39.38 | 37.50 | 28.70 | 39.57 | 45.71 | 33.96 | 38.64 | 44.03 | 39.73 | 34.48 | 36.08 | 38.95 | 28.63 | 30.66 | 36.26 | 32.12 | 32.22 |
| menthalactone | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d |
| γ-dodecalactone | 37.83 | 28.49 | 27.52 | 21.13 | 35.04 | 21.57 | 34.44 | 19.68 | 18.00 | 16.78 | 12.96 | 23.70 | 11.42 | 24.70 | 24.90 | 18.56 | 16.22 | 14.45 | 25.63 | 12.05 | 25.86 |
| zingerone | 0.505 | 2.26 | 0.690 | 0.579 | 0.334 | 1.58 | 1.26 | 0.693 | 0.541 | 0.547 | 0.767 | 0.491 | 1.84 | 0.979 | 0.771 | 0.597 | 0.532 | 0.921 | 0.525 | 1.82 | 1.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carlin, S.; Lotti, C.; Correggi, L.; Mattivi, F.; Arapitsas, P.; Vrhovšek, U. Measurement of the Effect of Accelerated Aging on the Aromatic Compounds of Gewürztraminer and Teroldego Wines, Using a SPE-GC-MS/MS Protocol. Metabolites 2022, 12, 180. https://doi.org/10.3390/metabo12020180
Carlin S, Lotti C, Correggi L, Mattivi F, Arapitsas P, Vrhovšek U. Measurement of the Effect of Accelerated Aging on the Aromatic Compounds of Gewürztraminer and Teroldego Wines, Using a SPE-GC-MS/MS Protocol. Metabolites. 2022; 12(2):180. https://doi.org/10.3390/metabo12020180
Chicago/Turabian StyleCarlin, Silvia, Cesare Lotti, Ludovica Correggi, Fulvio Mattivi, Panagiotis Arapitsas, and Urška Vrhovšek. 2022. "Measurement of the Effect of Accelerated Aging on the Aromatic Compounds of Gewürztraminer and Teroldego Wines, Using a SPE-GC-MS/MS Protocol" Metabolites 12, no. 2: 180. https://doi.org/10.3390/metabo12020180
APA StyleCarlin, S., Lotti, C., Correggi, L., Mattivi, F., Arapitsas, P., & Vrhovšek, U. (2022). Measurement of the Effect of Accelerated Aging on the Aromatic Compounds of Gewürztraminer and Teroldego Wines, Using a SPE-GC-MS/MS Protocol. Metabolites, 12(2), 180. https://doi.org/10.3390/metabo12020180