Exploring Thermal Sensitivities and Adaptations of Oxidative Phosphorylation Pathways
Abstract
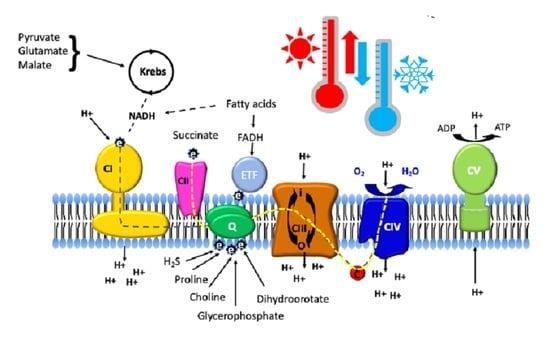
:1. Introduction
2. NADH and Succinate Pathways
3. Electron-Transferring Flavoprotein Pathway
4. Glycerophosphate Dehydrogenase
5. Dihydroorotate Dehydrogenase
6. Choline Dehydrogenase
7. Proline Dehydrogenase
8. Sulfide:Quinone Oxidoreductase
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Antão, L.H.; Bates, A.E.; Blowes, S.A.; Waldock, C.; Supp, S.R.; Magurran, A.E.; Dornelas, M.; Schipper, A.M. Temperature-related biodiversity change across temperate marine and terrestrial systems. Nat. Ecol. Evol. 2020, 4, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Blier, P.U.; Lemieux, H.; Pichaud, N. Holding our breath in our modern world: Will mitochondria keep the pace with global changes? Can. J. Zool. 2014, 92, 591–601. [Google Scholar] [CrossRef]
- Chung, D.J.; Schulte, P.M. Mitochondria and the thermal limits of ectotherms. J. Exp. Biol. 2020, 223, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Christen, F.; Desrosiers, V.; Dupont-Cyr, B.A.; Vandenberg, G.W.; Le Francois, N.R.; Tardif, J.C.; Dufresne, F.; Lamarre, S.G.; Blier, P.U. Thermal tolerance and thermal sensitivity of heart mitochondria: Mitochondrial integrity and ROS production. Free Radic. Biol. Med. 2018, 116, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Iftikar, F.I.; Hickey, A.J. Do mitochondria limit hot fish hearts? Understanding the role of mitochondrial function with heat stress in Notolabrus celidotus. PLoS ONE 2013, 8, e64120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pichaud, N.; Ballard, J.W.; Tanguay, R.M.; Blier, P.U. Thermal sensitivity of mitochondrial functions in permeabilized muscle fibers from two populations of Drosophila simulans with divergent mitotypes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R48–R59. [Google Scholar] [CrossRef] [Green Version]
- Pichaud, N.; Ballard, J.W.O.; Tanguay, R.M.; Blier, P.U. Naturally occurring mitochondrial DNA haplotypes exhibit metabolic differences: Insight into functional properties of mitochondria. Evolution 2012, 66, 3189–3197. [Google Scholar] [CrossRef]
- Blier, P.U.; Dufresne, F.; Burton, R.S. Natural selection and the evolution of mtDNA-encoded peptides: Evidence for intergenomic co-adaptation. Trends Genet. 2001, 17, 400–406. [Google Scholar] [CrossRef]
- Baris, T.Z.; Wagner, D.N.; Dayan, D.I.; Du, X.; Blier, P.U.; Pichaud, N.; Oleksiak, M.F.; Crawford, D.L. Evolved genetic and phenotypic differences due to mitochondrial-nuclear interactions. PLoS Genet 2017, 13, e1006517. [Google Scholar] [CrossRef] [Green Version]
- Lemieux, H.; Blier, P.U.; Gnaiger, E. Remodeling pathway control of oxidative phosphorylation by temperature in the heart. Sci. Rep. 2017, 7, 2840. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, R.; Purhonen, J.; Kallijärvi, J. The mitochondrial coenzyme Q junction and complex III: Biochemistry and pathophysiology. FEBS J. 2021, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Longo, N.; Frigeni, M.; Pasquali, M. Carnitine transport and fatty acid oxidation. Biochim. Biophys. Acta 2016, 1863, 2422–2435. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, H.; Tardif, J.C.; Dutil, J.D.; Blier, P.U. Thermal sensitivity of cardiac mitochondrial metabolism in an ectothermic species from a cold environment, Atlantic wolffish (Anarhichas lupus). J. Exp. Mar. Biol. Ecol. 2010, 384, 113–118. [Google Scholar] [CrossRef]
- Takeuchi, K.; Nakano, Y.; Kato, U.; Kaneda, M.; Aizu, M.; Awano, W.; Yonemura, S.; Kiyonaka, S.; Mori, Y.; Yamamoto, D.; et al. Changes in temperature preferences and energy homeostasis in dystroglycan mutants. Science 2009, 323, 1740–1743. [Google Scholar] [CrossRef] [PubMed]
- Blier, P.U.; Lemieux, H. The impact of the thermal sensitivity of cytochrome c oxidase on the respiration rate of Arctic charr red muscle mitochondria. J. Comp. Physiol. B 2001, 171, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, H.; Vazquez, E.J.; Fujioka, H.; Hoppel, C.L. Decrease in mitochondrial function in rat cardiac permeabilized fibers correlates with the aging phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 1157–1164. [Google Scholar] [CrossRef] [Green Version]
- Costa, L.E.; Boveris, A.; Koch, O.R.; Taquini, A.C. Liver and heart mitochondria in rats submitted to chronic hypobaric hypoxia. Am. J. Physiol.-Cell Physiol. 1988, 255, C123–C129. [Google Scholar] [CrossRef]
- Garait, B.; Couturier, K.; Servais, S.; Letexier, D.; Perrin, D.; Batandier, C.; Rouanet, J.L.; Sibille, B.; Rey, B.; Leverve, X.; et al. Fat intake reverses the beneficial effects of low caloric intake on skeletal muscle mitochondrial H2O2 production. Free Radic. Biol. Med. 2005, 39, 1249–1261. [Google Scholar] [CrossRef]
- Llesuy, S.; Evelson, P.; González-Flecha, B.; Peralta, J.; Carreras, M.C.; Poderoso, J.J.; Boveris, A. Oxidative stress in muscle and liver of rats with septic syndrome. Free Rad. Biol. Med. 1994, 16, 445–451. [Google Scholar] [CrossRef]
- Warren, B.E.; Lou, P.H.; Lucchinetti, E.; Zhang, L.; Clanachan, A.S.; Affolter, A.; Hersberger, M.; Zaugg, M.; Lemieux, H. Early mitochondrial dysfunction in glycolytic muscle, but not oxidative muscle, of the fructose-fed insulin-resistant rat. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E658–E667. [Google Scholar] [CrossRef] [Green Version]
- Aragonés, J.; Schneider, M.; Van Geyte, K.; Fraisl, P.; Dresselaers, T.; Mazzone, M.; Dirkx, R.; Zacchigna, S.; Lemieux, H.; Nam Ho Jeoung, N.H.; et al. Deficiency or inhibition of oxygen sensor Phd1 induces hypoxia tolerance by reprogramming basal metabolism. Nat. Genet. 2008, 40, 170–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gnaiger, E. Capacity of oxidative phosphorylation in human skeletal muscle. New perspectives of mitochondrial physiology. Int. J. Biochem. Cell Biol. 2009, 41, 1837–1845. [Google Scholar] [CrossRef] [PubMed]
- Gerber, L.; Clow, K.A.; Mark, F.C.; Gamperl, A.K. Improved mitochondrial function in salmon (Salmo salar) following high temperature acclimation suggests that there are cracks in the proverbial “ceiling”. Sci. Rep. 2020, 10, 21636. [Google Scholar] [CrossRef] [PubMed]
- Pichaud, N.; Ekström, A.; Breton, S.; Sundström, F.; Rowinski, P.; Blier, P.U.; Sandblom, E. Adjustments of cardiac mitochondrial phenotype in a warmer thermal habitat is associated with oxidative stress in European perch, Perca fluviatilis. Sci. Rep. 2020, 10, 17697. [Google Scholar] [CrossRef]
- Michaelsen, J.; Fago, A.; Bundgaard, A. High temperature impairs mitochondrial function in rainbow trout cardiac mitochondria. J. Exp. Biol. 2021, 224, jeb242382. [Google Scholar] [CrossRef]
- Iftikar, F.I.; Morash, A.J.; Cook, D.G.; Herbert, N.A.; Hickey, A.J. Temperature acclimation of mitochondria function from the hearts of a temperate wrasse (Notolabrus celidotus). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2015, 184, 46–55. [Google Scholar] [CrossRef]
- Dhingra, R.; Kirshenbaum, L.A. Succinate dehydrogenase/complex II activity obligatorily links mitochondrial reserve respiratory capacity to cell survival in cardiac myocytes. Cell Death Dis. 2015, 6, e1956. [Google Scholar] [CrossRef] [Green Version]
- Pfleger, J.; He, M.; Abdellatif, M. Mitochondrial complex II is a source of the reserve respiratory capacity that is regulated by metabolic sensors and promotes cell survival. Cell Death Dis. 2015, 6, e1835. [Google Scholar] [CrossRef] [Green Version]
- Treberg, J.R.; Quinlan, C.L.; Brand, M.D. Hydrogen peroxide efflux from muscle mitochondria underestimates matrix superoxide production-a correction using glutathione depletion. FEBS J. 2010, 277, 2766–2778. [Google Scholar] [CrossRef] [Green Version]
- St-Pierre, J.; Buckingham, J.A.; Roebuck, S.J.; Brand, M.D. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J. Biol. Chem. 2002, 277, 44784–44790. [Google Scholar] [CrossRef] [Green Version]
- Hansford, R.G.; Hogue, B.A.; Mildaziene, V. Dependence of H2O2 formation by rat heart mitochondria on substrate availability and donor age. J. Bioenerg. Biomembr. 1997, 29, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Lambert, A.J.; Brand, M.D. Superoxide production by NADH:ubiquinone oxidoreductase (complex I) depends on the pH gradient across the mitochondrial inner membrane. Biochem. J. 2004, 382, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, C.L.; Orr, A.L.; Perevoshchikova, I.V.; Treberg, J.R.; Ackrell, B.A.; Brand, M.D. Mitochondrial complex II can generate reactive oxygen species at high rates in both the forward and reverse reactions. J. Biol. Chem. 2012, 287, 27255–27264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siebels, I.; Dröse, S. Q-site inhibitor induced ROS production of mitochondrial complex II is attenuated by TCA cycle dicarboxylates. Biochim. Biophys. Acta 2013, 1827, 1156–1164. [Google Scholar] [CrossRef] [Green Version]
- Kluckova, K.; Sticha, M.; Cerny, J.; Mracek, T.; Dong, L.; Drahota, Z.; Gottlieb, E.; Neuzil, J.; Rohlena, J. Ubiquinone-binding site mutagenesis reveals the role of mitochondrial complex II in cell death initiation. Cell Death Dis. 2015, 6, e1749. [Google Scholar] [CrossRef] [Green Version]
- Blier, P.U.; Munro, D.; Degletagne, C.; Rodriguez, E.T. What modulates animal longevity? Fast and slow aging in bivalves as a model for the study of lifespan. Semin. Cell Dev. Biol. 2017, 70, 130–140. [Google Scholar] [CrossRef]
- Hunter-Manseau, F.; Desrosiers, V.; Le François, N.R.; Dufresne, F.; Detrich, H.W.; Nozais, C.; Blier, P.U. From Africa to Antarctica: Exploring the Metabolism of Fish Heart Mitochondria Across a Wide Thermal Range. Front. Physiol. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Munro, D.; Pichaud, N.; Paquin, F.; Kemeid, V.; Blier, P.U. Low hydrogen peroxide production in mitochondria of the long-lived Arctica islandica: Underlying mechanisms for slow aging. Aging Cell 2013, 12, 584–592. [Google Scholar] [CrossRef]
- Barja, G. Towards a unified mechanistic theory of aging. Exp. Gerontol. 2019, 124, 110627. [Google Scholar] [CrossRef]
- Lu, D.L.; Ma, Q.; Wang, J.; Li, L.Y.; Han, S.L.; Limbu, S.M.; Li, D.L.; Chen, L.Q.; Zhang, M.L.; Du, Z.Y. Fasting enhances cold resistance in fish through stimulating lipid catabolism and autophagy. J. Physiol. 2019, 597, 1585–1603. [Google Scholar] [CrossRef]
- Rodnick, K.J.; Sidell, B.D. Cold acclimation increases carnitine palmitoyltransferase I activity in oxidative muscle of striped bass. Am. J. Physiol. 1994, 266, R405–R412. [Google Scholar] [CrossRef] [PubMed]
- Guderley, H.; Gawlicka, A. Qualitative modification of muscle metabolic organization with thermal acclimation of rainbow trout, Oncorhynchus mykiss. Fish Physiol. Biochem. 1992, 10, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Kyprianou, T.D.; Portner, H.O.; Anestis, A.; Kostoglou, B.; Feidantsis, K.; Michaelidis, B. Metabolic and molecular stress responses of gilthead seam bream Sparus aurata during exposure to low ambient temperature: An analysis of mechanisms underlying the winter syndrome. J. Comp. Physiol. B 2010, 180, 1005–1018. [Google Scholar] [CrossRef] [PubMed]
- Crockett, E.L.; Sidell, B.D. Some pathways of energy metabolism are cold adapted in Antartic fishes. Physiol. Zool. 1990, 63, 472–488. [Google Scholar] [CrossRef]
- Lesser, M.P.; Kruse, V.A. Seasonal temperature compensation in the horse mussel, Modiolus modiolus: Metabolic enzymes, oxidative stress and heat shock proteins. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2004, 137, 495–504. [Google Scholar] [CrossRef]
- Rogers, K.D.; Thompson, M.B.; Seebacher, F. Beneficial acclimation: Sex specific thermal acclimation of metabolic capacity in the striped marsh frog (Limnodynastes peronii). J. Exp. Biol. 2007, 210, 2932–2938. [Google Scholar] [CrossRef] [Green Version]
- Gracey, A.Y.; Fraser, E.J.; Li, W.Z.; Fang, Y.X.; Taylor, R.R.; Rogers, J.; Brass, A.; Cossins, A.R. Coping with cold: An integrative, multitissue analysis of the transcriptome of a poikilothermic vertebrate. Proc. Nat. Acad. Sci. USA 2004, 101, 16970–16975. [Google Scholar] [CrossRef] [Green Version]
- Guderley, H.; Johnston, I.I. Plasticity of fish muscle mitochondria with thermal acclimation. J. Exp. Biol. 1996, 199, 1311–1317. [Google Scholar] [CrossRef]
- Hoskins, D.D.; Mackenzie, C.G. Solubilization and electron transfer flavoprtein requirement of mitochondrial sarcosine dehydrogenase and dimethylglycine dehydrogenase. J. Biol. Chem. 1961, 236, 177–183. [Google Scholar] [CrossRef]
- Ducker, G.S.; Rabinowitz, J.D. One-Carbon Metabolism in Health and Disease. Cell Metab. 2017, 25, 27–42. [Google Scholar] [CrossRef] [Green Version]
- Clare, C.E.; Brassington, A.H.; Kwong, W.Y.; Sinclair, K.D. One-Carbon Metabolism: Linking Nutritional Biochemistry to Epigenetic Programming of Long-Term Development. Annu. Rev. Anim. Biosci. 2019, 7, 263–287. [Google Scholar] [CrossRef] [PubMed]
- Abeles, R.H.; Frisell, W.R.; Mackenzie, C.G. A dual isotope effect in the enzymatic oxidation of deuteromethyl sarcosine. J. Biol. Chem. 1960, 235, 853–856. [Google Scholar] [CrossRef]
- Mackenzie, C.G.; Frisell, W.R. The metabolism of dimethylglycine by liver mitochondria. J. Biol. Chem. 1958, 232, 417–427. [Google Scholar] [CrossRef]
- Wittwer, A.J.; Wagner, C. Identification of the folate-binding proteins of rat liver mitochondria as dimethylglycine dehydrogenase and sarcosine dehydrogenase. Flavoprotein nature and enzymatic properties of the purified proteins. J. Biol. Chem. 1981, 256, 4109–4115. [Google Scholar] [CrossRef]
- Chung, D.J.; Sparagna, G.C.; Chicco, A.J.; Schulte, P.M. Patterns of mitochondrial membrane remodeling parallel functional adaptations to thermal stress. J. Exp. Biol 2018, 221, jeb174458. [Google Scholar] [CrossRef] [Green Version]
- Klingenberg, M. Localization of the glycerol-phosphate dehydrogenase in the outer phase of the mitochondrial inner membrane. Eur. J. Biochem. 1970, 13, 247–252. [Google Scholar] [CrossRef]
- Mráček, T.; Drahota, Z.; Houštěk, J. The function and the role of the mitochondrial glycerol-3-phosphate dehydrogenase in mammalian tissues. Biochim. Biophys. Acta 2013, 1827, 401–410. [Google Scholar] [CrossRef] [Green Version]
- Estabrook, R.W.; Sacktor, B. α-Glycerophosphate oxidase of flight muscle mitochondria. J. Biol. Chem. 1958, 233, 1014–1019. [Google Scholar] [CrossRef]
- Ghosh, R.K.; Ghosh, N.; De, S.; Ray, A.K.; Medda, A.K. Effect of l-triiodothyronine on the mitochondrial α-glycerophosphate dehydrogenase activity, mitochondrial and total protein contents of brain of Singi fish (Heteropneustes fossilis bloch). Neurochem. Int. 1983, 5, 635–640. [Google Scholar] [CrossRef]
- Medda, A.K.; Ghosh, R.K. Inhibitory influence of thiourea on brain of singi fish (Heteropneustes fossilis bloch) and subsequent recovery by l-triiodothyronine. Neurochem. Int. 1984, 6, 527–532. [Google Scholar] [CrossRef]
- Rigoulet, M.; Aguilaniu, H.; Avéret, N.; Bunoust, O.; Camougrand, N.; Grandier-Vazeille, X.; Larsson, C.; Pahlman, I.L.; Manon, S.; Gustafsson, L. Organization and regulation of the cytosolic NADH metabolism in the yeast Saccharomyces cerevisiae. Mol. Cell Biochem. 2004, 256–257, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Hess, R.; Pearse, A.G. Histochemical and homogenization studies of mitochondrial alpha-glycerophosphate dehydrogenase in the nervous system. Nature 1961, 191, 718–719. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.P.; Lardy, H.A. Influence of thyroid hormones on L-alpha-glycerophosphate dehydrogenases and other dehydrogenases in various organs of the rat. J. Biol. Chem. 1965, 240, 1427–1436. [Google Scholar] [CrossRef]
- Salganicoff, L.; Fukami, M.H. Energy metabolism of blood platelets. I. Isolation and properties of platelet mitochondria. Arch. Biochem. Biophys. 1972, 153, 726–735. [Google Scholar] [CrossRef]
- Schenkman, J.B.; Richert, D.A.; Westerfeld, W.W. α-Glycerophosphate dehydrogenase activity in rat spermatozoa. Endocrinology 1965, 76, 1055–1061. [Google Scholar] [CrossRef]
- Swierczyński, J.; Scislowski, P.; Aleksandrowicz, Z. High activity of alpha-glycerophosphate oxidation by human placental mitochondria. Biochim. Biophys. Acta 1976, 429, 46–54. [Google Scholar] [CrossRef]
- Bissell, M.J.; Rambeck, W.A.; White, R.C.; Bassham, J.A. Glycerol phosphate shuttle in virus-transformed cells in culture. Science 1976, 191, 856–858. [Google Scholar] [CrossRef] [Green Version]
- McDonald, A.E.; Pichaud, N.; Darveau, C.A. Alternative fuels contributing to mitochondrial electron transport: Importance of non-classical pathways in the diversity of animal metabolism. Comp. Biochem, Physiol, B Biochem, Mol. Biol. 2018, 224, 185–194. [Google Scholar] [CrossRef]
- Yeh, J.I.; Chinte, U.; Du, S. Structure of glycerol-3-phosphate dehydrogenase, an essential monotopic membrane enzyme involved in respiration and metabolism. Proc. Natl. Acad. Sci. USA 2008, 105, 3280–3285. [Google Scholar] [CrossRef] [Green Version]
- Xue, L.L.; Chen, H.H.; Jiang, J.G. Implications of glycerol metabolism for lipid production. Prog. Lipid. Res. 2017, 68, 12–25. [Google Scholar] [CrossRef]
- Orr, A.L.; Ashok, D.; Sarantos, M.R.; Ng, R.; Shi, T.; Gerencser, A.A.; Hughes, R.E.; Brand, M.D. Novel inhibitors of mitochondrial sn-glycerol 3-phosphate dehydrogenase. PLoS ONE 2014, 9, e89938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brisson, D.; Vohl, M.C.; St-Pierre, J.; Hudson, T.J.; Gaudet, D. Glycerol: A neglected variable in metabolic processes? Bioessays 2001, 23, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.B.; Gaviraghi, A.; Oliveira, M.F. Mitochondrial physiology in the major arbovirus vector Aedes aegypti: Substrate preferences and sexual differences define respiratory capacity and superoxide production. PLoS ONE 2015, 10, e0120600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez, E.; Hakkou, M.; Hagen, T.M.; Lemieux, H.; Blier, P.U. Divergences in the control of mitochondrial respiration are associated with life-span variation in marine bivalves. J. Gerontol. A Biol. Med. Sci. 2021, 76, 796–804. [Google Scholar] [CrossRef]
- Bettinazzi, S.; Rodríguez, E.; Milani, L.; Blier, P.U.; Breton, S. Metabolic remodelling associated with mtDNA: Insights into the adaptive value of doubly uniparental inheritance of mitochondria. Proc. Biol. Sci. 2019, 286, 20182708. [Google Scholar] [CrossRef] [Green Version]
- Paget, C.M.; Schwartz, J.M.; Delneri, D. Environmental systems biology of cold-tolerant phenotype in Saccharomyces species adapted to grow at different temperatures. Mol. Ecol. 2014, 23, 5241–5257. [Google Scholar] [CrossRef] [Green Version]
- Jørgensen, L.B.; Overgaard, J.; Hunter-Manseau, F.; Pichaud, N. Dramatic changes in mitochondrial substrate use at critically high temperatures: A comparative study using Drosophila. J. Exp. Biol. 2021, 224, jeb240960. [Google Scholar] [CrossRef]
- Lavington, E.; Cogni, R.; Kuczynski, C.; Koury, S.; Behrman, E.L.; O’Brien, K.R.; Schmidt, P.S.; Eanes, W.F. A small system--high-resolution study of metabolic adaptation in the central metabolic pathway to temperate climates in Drosophila melanogaster. Mol. Biol. Evol. 2014, 31, 2032–2041. [Google Scholar] [CrossRef] [Green Version]
- Koza, R.A.; Kozak, U.C.; Brown, L.J.; Leiter, E.H.; MacDonald, M.J.; Kozak, L.P. Sequence and tissue-dependent RNA expression of mouse FAD-linked glycerol-3-phosphate dehydrogenase. Arch. Biochem. Biophys. 1996, 336, 97–104. [Google Scholar] [CrossRef]
- DosSantos, R.A.; Alfadda, A.; Eto, K.; Kadowaki, T.; Silva, J.E. Evidence for a compensated thermogenic defect in transgenic mice lacking the mitochondrial glycerol-3-phosphate dehydrogenase gene. Endocrinology 2003, 144, 5469–5479. [Google Scholar] [CrossRef] [Green Version]
- Jacobsson, A.; Stadler, U.; Glotzer, M.A.; Kozak, L.P. Mitochondrial uncoupling protein from mouse brown fat. Molecular cloning, genetic mapping, and mRNA expression. J. Biol. Chem. 1985, 260, 16250–16254. [Google Scholar] [CrossRef]
- Ratner, P.L.; Fisher, M.; Burkart, D.; Cook, J.R.; Kozak, L.P. The role of mRNA levels and cellular localization in controlling sn-glycerol-3-phosphate dehydrogenase expression in tissues of the mouse. J. Biol. Chem. 1981, 256, 3576–3579. [Google Scholar] [CrossRef]
- Panadero, J.; Pallotti, C.; Rodríguez-Vargas, S.; Randez-Gil, F.; Prieto, J.A. A downshift in temperature activates the high osmolarity glycerol (HOG) pathway, which determines freeze tolerance in Saccharomyces cerevisiae. J. Biol. Chem. 2006, 281, 4638–4645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bortz, W.M.; Paul, P.; Haff, A.C.; Holmes, W.L. Glycerol turnover and oxidation in man. J. Clin. Invest. 1972, 51, 1537–1546. [Google Scholar] [CrossRef] [PubMed]
- Owen, O.E.; Felig, P.; Morgan, A.P.; Wahren, J.; Cahill, G.F. Liver and kidney metabolism during prolonged starvation. J. Clin. Invest. 1969, 48, 574–583. [Google Scholar] [CrossRef]
- de la Roche, M.; Tessier, S.N.; Storey, K.B. Structural and functional properties of glycerol-3-phosphate dehydrogenase from a mammalian hibernator. Protein J. 2012, 31, 109–119. [Google Scholar] [CrossRef]
- Berrada, W.; Naya, A.; Ouafik, L.; Bourhim, N. Effect of hibernation, thyroid hormones and dexamethasone on cytosolic and mitochondrial glycerol-3-phosphate dehydrogenase from jerboa (Jaculus orientalis). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2000, 125, 439–449. [Google Scholar] [CrossRef]
- Ruberto, A.A.; Childers, C.L.; Storey, K.B. Purification and properties of glycerol-3-phosphate dehydrogenase from the liver of the hibernating ground squirrel, Urocitellus richardsonii. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2016, 202, 48–55. [Google Scholar] [CrossRef]
- Reis, R.A.G.; Calil, F.A.; Feliciano, P.R.; Pinheiro, M.P.; Nonato, M.C. The dihydroorotate dehydrogenases: Past and present. Arch. Biochem. Biophys. 2017, 632, 175–191. [Google Scholar] [CrossRef]
- Nara, T.; Hshimoto, T.; Aoki, T. Evolutionary implications of the mosaic pyrimidine-biosynthetic pathway in eukaryotes. Gene 2000, 257, 209–222. [Google Scholar] [CrossRef]
- Zhang, J.; Terán, G.; Popa, M.; Madapura, H.; Ladds, M.; Lianoudaki, D.; Grünler, J.; Arsenian-Henriksson, M.; McCormack, E.; Rottenberg, M.E.; et al. DHODH inhibition modulates glucose metabolism and circulating GDF15, and improves metabolic balance. iScience 2021, 24, 102494. [Google Scholar] [CrossRef] [PubMed]
- Rawls, J.; Knecht, W.; Diekert, K.; Lill, R.; Löffler, M. Requirements for the mitochondrial import and localization of dihydroorotate dehydrogenase. Eur. J. Biochem. 2000, 267, 2079–2087. [Google Scholar] [CrossRef] [PubMed]
- Khutornenko, A.A.; Roudko, V.V.; Chernyak, B.V.; Vartapetian, A.B.; Chumakov, P.M.; Evstafieva, A.G. Pyrimidine biosynthesis links mitochondrial respiration to the p53 pathway. Proc. Natl. Acad. Sci. USA 2010, 107, 12828–12833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyazaki, Y.; Inaoka, D.K.; Shiba, T.; Saimoto, H.; Sakura, T.; Amalia, E.; Kido, Y.; Sakai, C.; Nakamura, M.; Moore, A.L.; et al. Selective Cytotoxicity of Dihydroorotate Dehydrogenase Inhibitors to Human Cancer Cells Under Hypoxia and Nutrient-Deprived Conditions. Front. Pharmacol. 2018, 9, 997. [Google Scholar] [CrossRef]
- Bader, B.; Knecht, W.; Fries, M.; Löffler, M. Expression, purification, and characterization of histidine-tagged rat and human flavoenzyme dihydroorotate dehydrogenase. Protein Expr. Purif. 1998, 13, 414–422. [Google Scholar] [CrossRef]
- Jones, S.W.; Penman, S.L.; French, N.S.; Park, B.K.; Chadwick, A.E. Investigating dihydroorotate dehydrogenase inhibitor mediated mitochondrial dysfunction in hepatic in vitro models. Toxicol In Vitro 2021, 72, 105096. [Google Scholar] [CrossRef]
- Sykes, D.B. The emergence of dihydroorotate dehydrogenase (DHODH) as a therapeutic target in acute myeloid leukemia. Expert Opin. Ther. Targets 2018, 22, 893–898. [Google Scholar] [CrossRef] [Green Version]
- Bajzikova, M.; Kovarova, J.; Coelho, A.R.; Boukalova, S.; Oh, S.; Rohlenova, K.; Svec, D.; Hubackova, S.; Endaya, B.; Judasova, K.; et al. Reactivation of dihydroorotate dehydrogenase-driven pyrimidine biosynthesis restores tumor growth of respiration-deficient cancer cells. Cell Metab. 2019, 29, 399–416. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Reyes, I.; Cardona, L.R.; Kong, H.; Vasan, K.; McElroy, G.S.; Werner, M.; Kihshen, H.; Reczek, C.R.; Weinberg, S.E.; Gao, P.; et al. Mitochondrial ubiquinol oxidation is necessary for tumour growth. Nature 2020, 585, 288–292. [Google Scholar] [CrossRef]
- Yatsuga, S.; Fujita, Y.; Ishii, A.; Fukumoto, Y.; Arahata, H.; Kakuma, T.; Kojima, T.; Ito, M.; Tanaka, M.; Saiki, R.; et al. Growth differentiation factor 15 as a useful biomarker for mitochondrial disorders. Ann. Neurol. 2015, 78, 814–823. [Google Scholar] [CrossRef] [Green Version]
- Boukalova, S.; Hubackova, S.; Milosevic, M.; Ezrova, Z.; Neuzil, J.; Rohlena, J. Dihydroorotate dehydrogenase in oxidative phosphorylation and cancer. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165759. [Google Scholar] [CrossRef]
- Fang, J.; Uchiumi, T.; Yagi, M.; Matsumoto, S.; Amamoto, R.; Takazaki, S.; Yamaza, H.; Nonaka, K.; Kang, D. Dihydro-orotate dehydrogenase is physically associated with the respiratory complex and its loss leads to mitochondrial dysfunction. Biosci. Rep. 2013, 33, e00021. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Jiang, S.; Xiao, Y.; He, Y.; Ren, T.; Jiang, L.; Liu, R.; Chen, Q. SOX2-dependent expression of dihydroorotate dehydrogenase regulates oral squamous cell carcinoma cell proliferation. Int. J. Oral Sci. 2021, 13, 3. [Google Scholar] [CrossRef]
- Costeira-Paulo, J.; Gault, J.; Popova, G.; Ladds, M.; van Leeuwen, I.M.M.; Sarr, M.; Olsson, A.; Lane, D.P.; Laín, S.; Marklund, E.G.; et al. Lipids Shape the Electron Acceptor-Binding Site of the Peripheral Membrane Protein Dihydroorotate Dehydrogenase. Cell Chem Biol 2018, 25, 309–317.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuge, H.; Nakano, Y.; Onishi, H.; Futamura, Y.; Ohashi, K. A novel purification and some properties of rat liver mitochondrial choline dehydrogenase. Biochim. Biophys. Acta 1980, 614, 274–284. [Google Scholar] [CrossRef]
- Gadda, G.; McAllister-Wilkins, E.E. Cloning, expression, and purification of choline dehydrogenase from the moderate halophile Halomonas elongata. Appl. Environ. Microbiol. 2003, 69, 2126–2132. [Google Scholar] [CrossRef] [Green Version]
- Grossman, E.B.; Hebert, S.C. Renal inner medullary choline dehydrogenase activity: Characterization and modulation. Am. J. Physiol. 1989, 256, F107–F112. [Google Scholar] [CrossRef]
- Miller, B.; Schmid, H.; Chen, T.J.; Schmolke, M.; Guder, W.G. Determination of choline dehydrogenase activity along the rat nephron. Biol. Chem. Hoppe Seyler 1996, 377, 129–137. [Google Scholar] [CrossRef]
- Boch, J.; Kempf, B.; Schmid, R.; Bremer, E. Synthesis of the osmoprotectant glycine betaine in Bacillus subtilis: Characterization of the gbsAB genes. J. Bacteriol. 1996, 178, 5121–5129. [Google Scholar] [CrossRef] [Green Version]
- Ikuta, S.; Imamura, S.; Misaki, H.; Horiuti, Y. Purification and characterization of choline oxidase from Arthrobacter globiformis. J. Biochem. 1977, 82, 1741–1749. [Google Scholar] [CrossRef]
- Lartillot, S. A simplified method of production of choline oxidase suitable for choline assay. Prep. Biochem. 1987, 17, 283–295. [Google Scholar] [PubMed]
- Rozwadowski, K.L.; Khachatourians, G.G.; Selvaraj, G. Choline oxidase, a catabolic enzyme in Arthrobacter pascens, facilitates adaptation to osmotic stress in Escherichia coli. J. Bacteriol. 1991, 173, 472–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burnet, M.; Lafontaine, P.J.; Hanson, A.D. Assay, Purification, and Partial Characterization of Choline Monooxygenase from Spinach. Plant Physiol. 1995, 108, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Rathinasabapathi, B.; Burnet, M.; Russell, B.L.; Gage, D.A.; Liao, P.C.; Nye, G.J.; Scott, P.; Golbeck, J.H.; Hanson, A.D. Choline monooxygenase, an unusual iron-sulfur enzyme catalyzing the first step of glycine betaine synthesis in plants: Prosthetic group characterization and cDNA cloning. Proc. Natl. Acad. Sci. USA 1997, 94, 3454–3458. [Google Scholar] [CrossRef] [Green Version]
- Figueroa-Soto, C.G.; Valenzuela-Soto, E.M. Glycine betaine rather than acting only as an osmolyte also plays a role as regulator in cellular metabolism. Biochimie 2018, 147, 89–97. [Google Scholar] [CrossRef]
- Pietruszko, R.; Chern, M. Betaine aldehyde dehydrogenase from rat liver mitochondrial matrix. Chem. Biol. Interact. 2001, 130–132, 193–199. [Google Scholar] [CrossRef]
- Wortmann, S.B.; Mayr, J.A. Choline-related-inherited metabolic diseases-A mini review. J. Inherit. Metab. Dis. 2019, 42, 237–242. [Google Scholar] [CrossRef] [Green Version]
- Rhodes, D.; Hanson, A.D. Quaternary ammonium and tertiary sulfonium compounds in higher plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1993, 44, 357–384. [Google Scholar] [CrossRef]
- Kempf, B.; Bremer, E. Uptake and synthesis of compatible solutes as microbial stress responses to high-osmolality environments. Arch. Microbiol. 1998, 170, 319–330. [Google Scholar] [CrossRef]
- Eklund, M.; Bauer, E.; Wamatu, J.; Mosenthin, R. Potential nutritional and physiological functions of betaine in livestock. Nutr. Res. Rev. 2005, 18, 31–48. [Google Scholar] [CrossRef] [Green Version]
- Kuznetsov, V.V.; Shevyakova, N.I. Stress responses of tobacco cells to high temperature and salinity. Proline accumulation and phosphorylation of polypeptides. Physiol. Plant. 1997, 100, 320–326. [Google Scholar] [CrossRef]
- Alia; Hayashi, H.; Sakamoto, A.; Murata, N. Enhancement of the tolerance of Arabidopsis to high temperatures by genetic engineering of the synthesis of glycinebetaine. Plant J. 1998, 16, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Alia; Mustardy, L.; Deshnium, P.; Ida, M.; Murata, N. Transformation of Arabidopsis thaliana with the codA gene for choline oxidase; accumulation of glycinebetaine and enhanced tolerance to salt and cold stress. Plant J. 1997, 12, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, A.; Valverde, R.; Alia; Chen, T.H.; Murata, N. Transformation of Arabidopsis with the codA gene for choline oxidase enhances freezing tolerance of plants. Plant J. 2000, 22, 449–453. [Google Scholar] [CrossRef]
- Alia; Hayashi, H.; Chen, T.H.H.; Murata, N. Transformation with a gene for choline oxidase enhances cold tolerance of Arabidopsis during germination and early growth. Plant Cell Environ. 1998, 21, 232–239. [Google Scholar] [CrossRef]
- Hayashi, H.; Alia; Sakamoto, A.; Nonaka, H.; Chen, T.H.H.; Murata, N. Enhanced germination under high-salt conditions of seeds of transgenic Arabidopsis with a bacterial gene (codA) for choline oxidase. J. Plant Res. 1998, 111, 357–362. [Google Scholar] [CrossRef]
- Bateman, J.B.; Evans, G.F.; Brown, P.R.; Gabriel, C.; Grant, E.H. Dielectric properties of the system bovine albumin: Urea: Betaine in aqueous solution. Phys. Med. Biol. 1992, 37, 175–182. [Google Scholar] [CrossRef]
- Xing, W.; Rajashekar, C.B. Glycine betaine involvement in freezing tolerance and water stress in Arabidopsis thaliana. Environ. Exp. Bot. 2001, 46, 21–28. [Google Scholar] [CrossRef]
- Allard, F.; Houde, M.; Krol, M.; Ivanov, A.; Huner, N.P.A.; Sarhan, F. Betaine improves freezing tolerance in wheat. Plant Cell Physiol. 1998, 39, 1194–1202. [Google Scholar] [CrossRef]
- Reaney, M.J.; Gusta, L.V. Factors Influencing the Induction of Freezing Tolerance by Abscisic Acid in Cell Suspension Cultures of Bromus inermis Leyss and Medicago sativa L. Plant Physiol. 1987, 83, 423–427. [Google Scholar] [CrossRef] [Green Version]
- Mantyla, E.; Lang, V.; Palva, E.T. Role of abscisic acid in drought-induced freezing tolerance, cold acclimation, and accumulation of LT178 and RAB18 proteins in Arabidopsis thaliana. Plant Physiol. 1995, 107, 141–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivero, R.M.; Ruiz, J.M.; Romero, L.M. Importance of N source on heat stress tolerance due to the accumulation of proline and quaternary ammonium compounds in tomato plants. Plant Biol. 2004, 6, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Naidu, B.P.; Paleg, L.G.; Aspinal, D.; Jennings, A.C.; Jones, G.P. Amino acid and glycine betaine accumulation in cold-stressed wheat seedlings. Phytochemistry 1991, 30, 407–409. [Google Scholar] [CrossRef]
- Che, T.M.; Aspinall, D.; Paleg, L.G. Stress metabolism. IV. Temperature stress and the accumulation of proline in barley and radish. J. Plant Physiol. 1974, 1, 87–97. [Google Scholar]
- Kuo, C.G.; Chen, H.M.; Ma, L.H. Effect of high temperature on proline content in tomato floral buds and leaves. J. Amer. Soc. Hort. Sci. 1986, 111, 746–750. [Google Scholar]
- Rathinasabapathi, B.; Sigua, C.; Ho, J.; Gage, D.A. Osmoprotectant beta-alanine betaine synthesis in the Plumbaginaceae: S-adenosyl-L-methionine dependent N-methylation of beta-alanine to its betaine is via N-methyl and N,N-dimethyl beta-alanines. Physiol. Plant. 2000, 109, 225–231. [Google Scholar] [CrossRef]
- Rathinasabapathi, B.; Gage, D.; Mackill, D.; Hanson, A. Cultivated and wild rices do not accumulate glycine-betaine due to deficiencies in two biosynthetic steps. Crop. Sci. 1993, 33, 534–538. [Google Scholar] [CrossRef]
- Park, S.; Choi, S.G.; Yoo, S.M.; Son, J.H.; Jung, Y.K. Choline dehydrogenase interacts with SQSTM1/p62 to recruit LC3 and stimulate mitophagy. Autophagy 2014, 10, 1906–1920. [Google Scholar] [CrossRef] [Green Version]
- Garza-Lombó, C.; Pappa, A.; Panayiotidis, M.I.; Franco, R. Redox homeostasis, oxidative stress and mitophagy. Mitochondrion 2020, 51, 105–117. [Google Scholar] [CrossRef]
- González Durán, E.; Cuaya, M.P.; Gutiérrez, M.V.; León, J.A. Effects of temperature and pH on the oxidative stress of benthic marine invertebrates. Biol. Bull. 2018, 45, 610–616. [Google Scholar] [CrossRef]
- Paital, B.; Chainy, G.B. Effects of temperature on complexes I and II mediated respiration, ROS generation and oxidative stress status in isolated gill mitochondria of the mud crab Scylla serrata. J. Therm. Biol. 2014, 41, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Servet, C.; Ghelis, T.; Richard, L.; Zilberstein, A.; Savoure, A. Proline dehydrogenase: A key enzyme in controlling cellular homeostasis. Front. Biosci. Landmark Ed. 2012, 17, 607–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hancock, C.N.; Liu, W.; Alvord, W.G.; Phang, J.M. Co-regulation of mitochondrial respiration by proline dehydrogenase/oxidase and succinate. Amino Acids 2016, 48, 859–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandhare, J.; Donald, S.P.; Cooper, S.K.; Phang, J.M. Regulation and function of proline oxidase under nutrient stress. J. Cell Biochem. 2009, 107, 759–768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagedorn, C.H.; Phang, J.M. Transfer of reducing equivalents into mitochondria by the interconversions of proline and delta 1-pyrroline-5-carboxylate. Arch. Biochem. Biophys. 1983, 225, 95–101. [Google Scholar] [CrossRef]
- Phang, J.M. The regulatory functions of proline and pyrroline-5-carboxylic acid. Curr. Top. Cell Regul. 1985, 25, 91–132. [Google Scholar]
- Donald, S.P.; Sun, X.Y.; Hu, C.A.; Yu, J.; Mei, J.M.; Valle, D.; Phang, J.M. Proline oxidase, encoded by p53-induced gene-6, catalyzes the generation of proline-dependent reactive oxygen species. Cancer Res. 2001, 61, 1810–1815. [Google Scholar]
- Hu, C.A.; Donald, S.P.; Yu, J.; Lin, W.W.; Liu, Z.; Steel, G.; Obie, C.; Valle, D.; Phang, J.M. Overexpression of proline oxidase induces proline-dependent and mitochondria-mediated apoptosis. Mol. Cell Biochem. 2007, 295, 85–92. [Google Scholar] [CrossRef]
- Liu, Y.; Borchert, G.L.; Donald, S.P.; Surazynski, A.; Hu, C.A.; Weydert, C.J.; Oberley, L.W.; Phang, J.M. MnSOD inhibits proline oxidase-induced apoptosis in colorectal cancer cells. Carcinogenesis 2005, 26, 1335–1342. [Google Scholar] [CrossRef]
- Natarajan, S.K.; Zhu, W.; Liang, X.; Zhang, L.; Demers, A.J.; Zimmerman, M.C.; Simpson, M.A.; Becker, D.F. Proline dehydrogenase is essential for proline protection against hydrogen peroxide-induced cell death. Free Radic. Biol. Med. 2012, 53, 1181–1191. [Google Scholar] [CrossRef] [Green Version]
- Zarse, K.; Schmeisser, S.; Groth, M.; Priebe, S.; Beuster, G.; Kuhlow, D.; Guthke, R.; Platzer, M.; Kahn, C.R.; Ristow, M. Impaired insulin/IGF1 signaling extends life span by promoting mitochondrial L-proline catabolism to induce a transient ROS signal. Cell Metab. 2012, 15, 451–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishimura, A.; Yoshikawa, Y.; Ichikawa, K.; Takemoto, T.; Tanahashi, R.; Takagi, H. Longevity regulation by proline oxidation in yeast. Microorganisms 2021, 9, 1650. [Google Scholar] [CrossRef] [PubMed]
- Teulier, L.; Weber, J.M.; Crevier, J.; Darveau, C.A. Proline as a fuel for insect flight: Enhancing carbohydrate oxidation in hymenopterans. Proc. Biol. Sci. 2016, 283, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olembo, N.K.; Pearson, D.J. Changes in the contents of intermediates of proline and carbohydrate metabolism in flight muscle of the tsetse fly Glossina morsitans and the fleshfly Sarcophaga tibialis. Insect Biochem. 1982, 12, 657–662. [Google Scholar] [CrossRef]
- Giulivi, C.; Ross-Inta, C.; Horton, A.A.; Luckhart, S. Metabolic pathways in Anopheles stephensi mitochondria. Biochem. J. 2008, 415, 309–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldstrohm, D.A.; Pennington, J.E.; Wells, M.A. The role of hemolymph proline as a nitrogen sink during blood meal digestion by the mosquito Aedes aegypti. J. Insect Physiol. 2003, 49, 115–121. [Google Scholar] [CrossRef]
- Scaraffia, P.Y.; Wells, M.A. Proline can be utilized as an energy substrate during flight of Aedes aegypti females. J. Insect Physiol. 2003, 49, 591–601. [Google Scholar] [CrossRef]
- Bursell, E. Substrates of oxidative metabolism in dipteran flight muscle. Comp. Biochem. Physiol. B 1975, 52, 235–238. [Google Scholar] [CrossRef]
- Bursell, E. Aspects of the metabolism of amino acids in the tsetse fly, Glossina (Diptera). J. Insect Physiol. 1963, 9, 439–452. [Google Scholar] [CrossRef]
- Ballantyne, J.S.; Storey, K.B. Mitochondria from the ventricle of the marine clam, Mercenaria mercenaria: Substrate preferences and effects of pH and salt concentration on proline oxidation. Comp. Biochem. Physiol. B Comp. Biochem. 1983, 76, 133–138. [Google Scholar] [CrossRef]
- Gäde, G.; Auerswald, L. Beetles’ choice–proline for energy output: Control by AKHs. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2002, 132, 117–129. [Google Scholar] [CrossRef]
- Storey, K.B.; Baust, J.G.; Storey, J.M. Intermediary metabolism during low temperature acclimation in the overwintering gall fly larva, Eurosta solidaginis. J. Comp. Physiol. B 1981, 144, 183–190. [Google Scholar] [CrossRef]
- Morgan, T.D.; Chippendale, G.M. Free amino acids of the haemolymph of the southwestern corn borer and the European corn borer in relation to their diapause. J. Insect Physiol. 1983, 29, 735–740. [Google Scholar] [CrossRef]
- Marchese, L.; Olavarria, K.; Mantilla, B.S.; Avila, C.C.; Souza, R.O.O.; Damasceno, F.S.; Elias, M.C.; Silber, A.M. Trypanosoma cruzi synthesizes proline via a Δ1-pyrroline-5-carboxylate reductase whose activity is fine-tuned by NADPH cytosolic pools. Biochem. J. 2020, 477, 1827–1845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magdaleno, A.; Ahn, I.Y.; Paes, L.S.; Silber, A.M. Actions of a proline analogue, L-thiazolidine-4-carboxylic acid (T4C), on Trypanosoma cruzi. PLoS ONE 2009, 4, e4534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terburgh, K.; Lindeque, Z.; Mason, S.; van der Westhuizen, F.; Louw, R. Metabolomics of Ndufs4(−/−) skeletal muscle: Adaptive mechanisms converge at the ubiquinone-cycle. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 98–106. [Google Scholar] [CrossRef]
- Dionisio-Sese, M.L.; Shono, M.; Tobita, S. Effects of proline and betaine on heat inactivation of ribulose-1,5-bisphosphate carboxylase/oxygenase in crude extracts of rice seedlings. Photosynthetica 1999, 36, 557–563. [Google Scholar] [CrossRef]
- Chang, Y.C.; Lee, T.M. High temperature-induced free proline accumulation in Gracilaria tenuistipitata (Rhodophyta). Bot. Bull. Acad. Sin. 1999, 40, 289–294. [Google Scholar]
- Khan, A.; Ahmad, M.; Ahmed, M.; Iftikhar Hussain, M. Rising atmospheric temperature impact on wheat and thermotolerance strategies. Plants 2020, 10, 43. [Google Scholar] [CrossRef]
- Schobert, B.; Tschesche, H. Unusual solution properties of proline and its interaction with proteins. Biochim. Biophys. Acta 1978, 541, 270–277. [Google Scholar] [CrossRef]
- Paleg, L.G.; Douglas, T.J.; Van Daal, A.; Keech, D.B. Proline, betaine and other organic solutes protect enzymes against heat inactivation. Austr. J. Plant Physiol. 1981, 8, 107–114. [Google Scholar] [CrossRef]
- Charest, C.; Phan, C.T. Cold acclimation of wheat: Properties of enzymes involved in proline metabolism. Physiol. Plant. 1990, 80, 159–168. [Google Scholar] [CrossRef]
- Sarkar, D.; Bhowmik, P.C.; Kwon, Y.I.; Shetty, K. Clonal response to cold tolerance in creeping bentgrass and role of proline-associated pentose phosphate pathway. Bioresour. Technol. 2009, 100, 5332–5339. [Google Scholar] [CrossRef] [PubMed]
- Ismail; Hamayun, M.; Hussain, A.; Afzal Khan, S.; Iqbal, A.; Lee, I.J. Aspergillus flavus promoted the growth of soybean and sunflower seedlings at elevated temperature. BioMed Res. Int. 2019, 2019, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouillaud, F.; Blachier, F. Mitochondria and sulfide: A very old story of poisoning, feeding, and signaling? Antioxid. Redox. Signal. 2011, 15, 379–391. [Google Scholar] [CrossRef]
- Evans, C.L. The toxicity of hydrogen sulphide and other sulphides. Q J. Exp. Physiol. Cogn. Med. Sci. 1967, 52, 231–248. [Google Scholar] [CrossRef]
- Nicholls, P. The effect of sulphide on cytochrome aa3. Isosteric and allosteric shifts of the reduced alpha-peak. Biochim. Biophys. Acta 1975, 396, 24–35. [Google Scholar] [CrossRef]
- Julian, D.; April, K.L.; Patel, S.; Stein, J.R.; Wohlgemuth, S.E. Mitochondrial depolarization following hydrogen sulfide exposure in erythrocytes from a sulfide-tolerant marine invertebrate. J. Exp. Biol. 2005, 208, 4109–4122. [Google Scholar] [CrossRef] [Green Version]
- Landry, A.P.; Ballou, D.P.; Banerjee, R. Hydrogen Sulfide Oxidation by Sulfide Quinone Oxidoreductase. Chembiochem 2021, 22, 949–960. [Google Scholar] [CrossRef]
- Kimura, H. Hydrogen sulfide: From brain to gut. Antioxid. Redox. Signal. 2010, 12, 1111–1123. [Google Scholar] [CrossRef]
- Módis, K.; Ju, Y.; Ahmad, A.; Untereiner, A.A.; Altaany, Z.; Wu, L.; Szabo, C.; Wang, R. S-sulfhydration of ATP synthase by hydrogen sulfide stimulates mitochondrial bioenergetics. Pharmacol. Res. 2016, 113, 116–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Wang, M.; Zhong, Z.; Chen, H.; Wang, H.; Zhou, L.; Cao, L.; Fu, L.; Zhang, H.; Lian, C.; et al. Adaption to hydrogen sulfide-rich environments: Strategies for active detoxification in deep-sea symbiotic mussels, Gigantidas platifrons. Sci. Total Environ. 2022, 804, 150054. [Google Scholar] [CrossRef]
- Icoglu Aksakal, F.; Ciltas, A. The impact of ultraviolet B (UV-B) radiation in combination with different temperatures in the early life stage of zebrafish (Danio rerio). Photochem. Photobiol. Sci. 2018, 17, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Li, L.; Li, Q.; He, X.; Cui, Z. Transcriptomic characterization of temperature stress responses in larval zebrafish. PLoS ONE 2012, 7, e37209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, Y.; Yan, J.; Song, G.; Li, X.; Li, X.; Li, Q.; Cui, Z. Transcriptional events co-regulated by hypoxia and cold stresses in zebrafish larvae. BMC Genom. 2015, 16, 385. [Google Scholar] [CrossRef] [Green Version]
- Feugere, L.; Scott, V.F.; Rodriguez-Barucg, Q.; Beltran-Alvarez, P.; Wollenberg Valero, K.C. Thermal stress induces a positive phenotypic and molecular feedback loop in zebrafish embryos. J. Therm. Biol. 2021, 102, 103114. [Google Scholar] [CrossRef]
- Lemieux, H.; Semsroth, S.; Antretter, H.; Höfer, D.; Gnaiger, E. Mitochondrial respiratory control and early defects of oxidative phosphorylation in the failing human heart. Int. J. Biochem. Cell Biol. 2011, 43, 1729–1738. [Google Scholar] [CrossRef]
- Lemieux, H.; Warren, B. An animal model to study human muscular diseases involving mitochondrial oxidative phosphorylation. J. Bioenerg. Biomembr. 2012, 44, 503–512. [Google Scholar] [CrossRef]
- Wiens, L.; Banh, S.; Sotiri, E.; Jastroch, M.; Block, B.A.; Brand, M.D.; Treberg, J.R. Comparison of mitochondrial reactive oxygen species production of ectothermic and endothermic fish muscle. Front. Physiol. 2017, 8, 704. [Google Scholar] [CrossRef] [Green Version]
- Munro, D.; Treberg, J.R. A radical shift in perspective: Mitochondria as regulators of reactive oxygen species. J. Exp. Biol. 2017, 220, 1170–1180. [Google Scholar] [CrossRef] [Green Version]
- Thoral, E.; Roussel, D.; Chinopoulos, C.; Teulier, L.; Salin, K. Low oxygen levels can help to prevent the detrimental effect of acute warming on mitochondrial efficiency in fish. Biol. Lett. 2021, 17, 20200759. [Google Scholar] [CrossRef] [PubMed]
- Salin, K.; Villasevil, E.M.; Anderson, G.J.; Selman, C.; Chinopoulos, C.; Metcalfe, N.B. The RCR and ATP/O indices can give contradictory messages about mitochondrial efficiency. Integr. Comp. Biol. 2018, 58, 486–494. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lemieux, H.; Blier, P.U. Exploring Thermal Sensitivities and Adaptations of Oxidative Phosphorylation Pathways. Metabolites 2022, 12, 360. https://doi.org/10.3390/metabo12040360
Lemieux H, Blier PU. Exploring Thermal Sensitivities and Adaptations of Oxidative Phosphorylation Pathways. Metabolites. 2022; 12(4):360. https://doi.org/10.3390/metabo12040360
Chicago/Turabian StyleLemieux, Hélène, and Pierre U. Blier. 2022. "Exploring Thermal Sensitivities and Adaptations of Oxidative Phosphorylation Pathways" Metabolites 12, no. 4: 360. https://doi.org/10.3390/metabo12040360
APA StyleLemieux, H., & Blier, P. U. (2022). Exploring Thermal Sensitivities and Adaptations of Oxidative Phosphorylation Pathways. Metabolites, 12(4), 360. https://doi.org/10.3390/metabo12040360