Effects of Water Stress, Defoliation and Crop Thinning on Vitis vinifera L. cv. Solaris: Part I: Plant Responses, Fruit Development and Fruit Quality
Abstract
:1. Introduction
2. Results
2.1. Climate
2.2. Impact of Water Deficit (WD) on the Vegetative Parameters of Vines
2.3. Impact of Water Deficit, Defoliation and Crop-Thinning on Yield and Cluster Weight
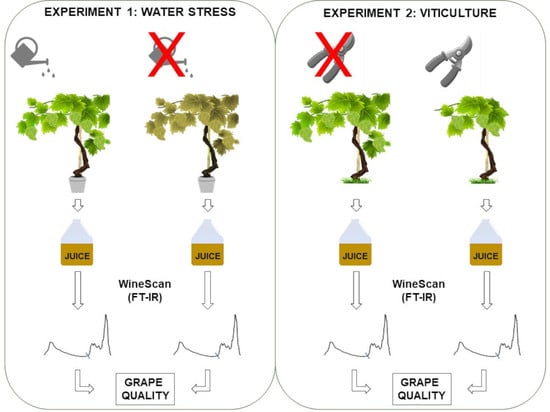
2.4. Analysis of Juice Samples by FT-IR (WineScan)
2.4.1. Impact of WD as Measured at Harvest
2.4.2. Impact of WD on Fruit Development
2.4.3. Impact of Def and CT as Measured at Harvest
2.4.4. Impact of Def and CT during Fruit Development Relative to WD
3. Discussion
3.1. Observations on Yield and Vegetative Parameters
3.2. Observations on Defoliation
3.3. Observations on Crop-Thinning
3.4. Observations on Water Deficit
3.5. Observations on Potassium and Nutrients/Amino Compounds
4. Materials and Methods
4.1. Experimental Design
4.1.1. Open Screenhouse Experiment
4.1.2. Vineyard Experiments
4.2. Preparation and Collection of Juice Samples
4.3. WineScan Analysis
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fawzy, S.; Osman, A.I.; Doran, J.; Rooney, D.W. Strategies for mitigation of climate change: A review. Environ. Chem. Lett. 2020, 18, 2069–2094. [Google Scholar] [CrossRef]
- Schernewski, G.; Hofstede, J.; Neumann, T. Global Change and Baltic Coastal Zones; Springer: Dordrecht, The Netherlands; New York, NY, USA, 2011; ISBN 9789400703995. [Google Scholar]
- Becker, J.R.; Toldam-Andersen, T.B. The wine industry in Denmark. Development 2012, 90, 100. [Google Scholar]
- Landbrugsstyrelsen: Notat-Opgørelse af Afgrødefordeling 2021. Ministry of Food, Agriculture and Fisheries. J.nr. 21-22120-000143. Available online: https://lbst.dk/fileadmin/user_upload/NaturErhverv/Filer/Tilskud/Arealtilskud/Direkte_stoette_-_grundbetaling_mm/2021/Opgoerelse_af_afgroedefordelingen_2021.pdf (accessed on 8 February 2022).
- Gladstone, J. Viticulture and Environment, 2nd ed.; Trivium Press PTY Ltd.: Adelaide, Australia, 2016; ISBN 978-0-9945016-1-5. [Google Scholar]
- Boso, S.; Kassemeyer, H.H. Different susceptibility of European grapevine cultivars for downy mildew. Vitis J. Grapevine Res. 2008, 47, 39–49. [Google Scholar]
- Dudenhöffer, J.; Schwander, F.; Töpfer, R.; Zyprian, E. Sequence analysis of loci rpv10 and rpv3 for resistance against grapevine downy mildew (Plasmopara viticola). Acta Hortic. 2015, 1082, 69–72. [Google Scholar] [CrossRef] [Green Version]
- Lisek, J.; Lisek, A. Cold Hardiness of Primary Buds of Wine and Table Grape Cultivars in Poland. S. Afr. J. Enol. Vitic. 2020, 41, 189–196. [Google Scholar] [CrossRef]
- Liu, J.; Toldam-Andersen, T.B.; Petersen, M.A.; Zhang, S.; Arneborg, N.; Bredie, W.L.P. Instrumental and sensory characterisation of Solaris white wines in Denmark. Food Chem. 2015, 166, 133–142. [Google Scholar] [CrossRef]
- Zhang, S.; Petersen, M.A.; Liu, J.; Toldam-Andersen, T.B. Influence of pre-fermentation treatments on wine volatile and sensory profile of the new disease tolerant cultivar Solaris. Molecules 2015, 20, 21609–21625. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Arneborg, N.; Toldam-Andersen, T.B.; Zhang, S.; Petersen, M.A.; Bredie, W.L.P. Impact of sequential co-culture fermentations on flavour characters of Solaris wines. Eur. Food Res. Technol. 2017, 243, 437–445. [Google Scholar] [CrossRef]
- Chenot, C.; Briffoz, L.; Lomartire, A.; Collin, S. Occurrence of Ehrlich-Derived and Varietal Polyfunctional Thiols in Belgian White Wines Made from Chardonnay and Solaris Grapes. J. Agric. Food Chem. 2020, 68, 10310–10317. [Google Scholar] [CrossRef]
- Nordmark, L.; Lindén, J.; Skjöldebrand, C.; Hansson, H. The Nordic Light Terroir. Acta Hortic. 2016, 1115, 189–193. [Google Scholar] [CrossRef]
- Porro, D.; Wolf, M.; Pedò, S. Evaluation of mechanical properties of berries on resistant or tolerant varieties of grapevine. BIO Web Conf. 2019, 13, 01005. [Google Scholar] [CrossRef]
- Pedò, S.; Bottura, M.; Porro, D. Development, yield potential and nutritional aspects of resistant grapevine varieties in Trentino Alto Adige. BIO Web Conf. 2019, 13, 02004. [Google Scholar] [CrossRef]
- Reynolds, A.G. Viticultural and Vineyard Management Practices and Their Effects on Grape and Wine Quality; Woodhead Publishing Limited: Cambridge, MA, USA, 2010; ISBN 9781845694845. [Google Scholar]
- Freeman, B.M.; Kliewer, W.M. Effect of irrigation, crop level and potassium fertilization on Carignane vines. II. Grape and wine quality. Am. J. Enol. Vitic. 1983, 34, 197–207. [Google Scholar]
- Esteban, M.A.; Villanueva, M.J.; Lissarrague, J.R. Effect of irrigation on changes in berry composition of Tempranillo during maturation. Sugars, organic acids, and mineral elements. Am. J. Enol. Vitic. 1999, 50, 418–434. [Google Scholar]
- Intrigliolo, D.S.; Castel, J.R. Interactive effects of deficit irrigation and shoot and cluster thinning on grapevine cv. Tempranillo. Water relations, vine performance and berry and wine composition. Irrig. Sci. 2011, 29, 443–454. [Google Scholar] [CrossRef]
- Wenter, A.; Zanotelli, D.; Montagnani, L.; Tagliavini, M.; Andreotti, C. Effect of different timings and intensities of water stress on yield and berry composition of grapevine (cv. Sauvignon blanc) in a mountain environment. Sci. Hortic. 2018, 236, 137–145. [Google Scholar] [CrossRef]
- Cappelen, J. Denmark—DMI Historical Climate Data Collection 1768–2020; DMI Report 21-02. 2021. ISSN 2445-9127. Available online: https://www.dmi.dk/publikationer/ (accessed on 3 March 2022).
- Greenspan, M.; Shackel, K.A.; Matthews, M.A. Developmental changes in the diurnal water budget of the grape berry exposed to water deficits. Plant Cell Environ. 1994, 17, 811–820. [Google Scholar] [CrossRef]
- Moreno, D.; Valdés, E.; Uriarte, D.; Gamero, E.; Talaverano, I.; Vilanova, M. Early leaf removal applied in warm climatic conditions: Impact on Tempranillo wine volatiles. Food Res. Int. 2017, 98, 50–58. [Google Scholar] [CrossRef]
- Palliotti, A.; Gardi, T.; Berrios, J.G.; Civardi, S.; Poni, S. Early source limitation as a tool for yield control and wine quality improvement in a high-yielding red Vitis vinifera L. cultivar. Sci. Hortic. 2012, 145, 10–16. [Google Scholar] [CrossRef]
- Sivilotti, P.; Herrera, J.C.; Lisjak, K.; Cesnik, H.B.; Sabbatini, P.; Peterlunger, E.; Castellarin, S.D. Impact of leaf removal, applied before and after flowering on anthocyanin tannin and methoxyyrazine concentrations in Merlot (Vitis vinifera L.) grapes and wines. J. Agric. Food Chem. 2016, 64, 4487–4496. [Google Scholar] [CrossRef] [Green Version]
- Tittmann, S.; Stoeber, V.; Stoll, M. Impact of defoliation on leaf and berry compounds of Vitis vinifera L. cv. Riesling investigated using non-destructive methods. In Proceedings of the International Viticulture and Enology Society IVES Conference Series, Plant Management across Different Terroirs, Online Conference, 10–14 July 2016; pp. 278–282. [Google Scholar]
- Bubola, M.; Peršurić, D.; Ganić, K.K. Impact of cluster thinning on productive characteristics and wine phenolic composition of cv. Merlot. J. Food Agric. Environ. 2011, 9, 36–39. [Google Scholar]
- Bell, S.J.; Henschke, P.A. Implications of nitrogen nutrition for grapes, fermentation and wine. Aust. J. Grape Wine Res. 2005, 11, 242–295. [Google Scholar] [CrossRef]
- Tardaguila, J.; de Toda, F.M.; Poni, S.; Diago, M.P. Impact of early leaf removal on yield and fruit and wine composition of Vitis vinifera L. Graciano and Carignan. Am. J. Enol. Vitic. 2010, 61, 372–381. [Google Scholar]
- Zhang, P.; Wu, X.; Needs, S.; Liu, D.; Fuentes, S.; Howell, K. The Influence of Apical and Basal Defoliation on the Canopy Structure and Biochemical Composition of Vitis vinifera cv. Shiraz Grapes and Wine. Front. Chem. 2017, 5, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabir, A.; Kafkas, E.; Tangolar, S. Distribution of major sugars, acids, and total phenols in juice of five grapevine (Vitis spp.) cultivars at different stages of berry development. Span. J. Agric. Res. 2010, 8, 425. [Google Scholar] [CrossRef]
- Lakso, A.N.; Kliewer, W.M. The Influence of Temperature on Malic Acid Metabolism in Grape Berries. Plant Physiol. 1975, 56, 370–372. [Google Scholar] [CrossRef] [Green Version]
- Melino, V.J.; Hayes, M.; Soole, K.L.; Ford, C.M. The role of light in the regulation of ascorbate metabolism during berry development in the cultivated grapevine Vitis vinifera L. J. Sci. Food Agric. 2011, 91, 1712–1721. [Google Scholar] [CrossRef]
- Etchebarne, F.; Ojeda, H.; Hunter, J. Leaf: Fruit Ratio and Vine Water Status Effects on Grenache Noir (Vitis vinifera L.) Berry Composition: Water, Sugar, Organic Acids and Cations. S. Afr. J. Enol. Vitic. 2010, 31, 106–115. [Google Scholar] [CrossRef] [Green Version]
- Gil, M.; Esteruelas, M.; González, E.; Kontoudakis, N.; Jiménez, J.; Fort, F.; Canals, J.M.; Hermosín-Gutiérrez, I.; Zamora, F. Effect of two different treatments for reducing grape yield in Vitis vinifera cv. Syrah on wine composition and quality: Berry thinning versus cluster thinning. J. Agric. Food Chem. 2013, 61, 4968–4978. [Google Scholar] [CrossRef]
- Petrie, P.R.; Clingeleffer, P.R. Crop thinning (hand versus mechanical), grape maturity and anthocyanin concentration: Outcomes from irrigated Cabernet Sauvignon (Vitis vinifera L.) in a warm climate. Aust. J. Grape Wine Res. 2006, 12, 21–29. [Google Scholar] [CrossRef]
- Kliewer, W.M.; Dokoozlian, N.K. Leaf area/crop weight ratios of grapevines: Influence on fruit composition and wine quality. Am. J. Enol. Vitic. 2005, 56, 170–181. [Google Scholar]
- Hartge, K.H.; Wiebe, H.J. Energetic State of Water in Plant and Soil, Its Influence on Crop Yield and Determination Methods. Die Gart. 1977, 42, 71–76. [Google Scholar]
- Dry, P.R.; Loveys, B.R.; Botting, D.; During, H. Effects of partial root-zone drying on grapevine vigour, yield, composition of fruit and use of water. In Proceedings of the 9th Australian Wine Industry Technical Conference, Adelaide, Australia, 16–19 July 1995; pp. 128–131. [Google Scholar]
- Bindon, K.; Dry, P.; Loveys, B. Influence of partial rootzone drying on the composition and accumulation of anthocyanins in grape berries (Vitis vinifera cv. Cabernet Sauvignon). FTIR spectroscopy for grape and wine analysis. Aust. J. Grape Wine Res. 2008, 14, 91–103. [Google Scholar] [CrossRef]
- Reynolds, A.G.; Naylor, A.P. ‘Pinot noir’ and ‘Riesling’ Grapevines Respond to Water Stress Duration and Soil Water-holding Capacity. HortScience 1994, 29, 1505–1510. [Google Scholar] [CrossRef] [Green Version]
- Bauer, R.; Nieuwoudt, H.; Bauer, F.F.; Koch, K.R.; Esbensen, K.H. FTIR Spectroscopy for grape and wine Analysis. Anal. Chem. 2008, 80, 1371–1379. [Google Scholar] [CrossRef] [Green Version]



| Time Period of Measurement | |||
|---|---|---|---|
| Plant Group: | Phase 1 | Phase 2 | Phase 3 |
| Control | 24.1 ± 4.9 a | 24.2 ± 5.9 a | 27.3 ± 6.3 a |
| Early stress | 9.6 ± 2.9 b | 19.9 ± 6.8 b | 22.9 ± 7.4 b |
| Mid stress | - | 7.1 ± 3.4 c | 23.1 ± 3.9 b |
| Late stress | - | 20.8 ± 7.6 b | 9.3 ± 2.9 c |
| Time Period of Measurement | |||
|---|---|---|---|
| Plant Group: | Phase 1 | Phase 2 | Phase 3 |
| Control | - | −0.68 ± 0.16 a | −0.61 ± 0.07 a |
| Early stress | - | −0.72 ± 0.15 a | −0.64 ± 0.06 a |
| Mid stress | - | −1.2 ± 0.19 b | −0.71 ± 0.08 a |
| Late stress | - | −0.70 ± 0.07 a | −1.03 ± 0.25 b |
| PVP | Unit | SC, Control | SC, Stress | DC, Control | DC, Stress |
|---|---|---|---|---|---|
| P shoot, L | cm/shoot | 142 ± 14.8 a | 127 ± 13.7 a,b | 114 ± 12.9 b,c | 101 ± 15.3 c |
| P shoot, N | #/plant | 15 ± 1.0 b | 16.5 ± 0.7 b | 22.5 ± 2.1 a | 25 ± 3.7 a |
| P shoot, FW | g/plant | 1083 ± 63 a | 868 ± 7 b,c | 945 ± 87 b | 821 ± 106 c |
| P leaves, N | #/plant | 219 ± 27.5 b | 234 ± 72 b | 323 ± 15.7 a | 337 ± 30.2 a |
| P leaves, FW | g/plant | 1488 ± 109 a | 1455 ± 178 a | 1667 ± 134 a | 1657 ± 162 a |
| P leaf, A | m2/plant | 5.4 ± 0.72 b | 5.3 ± 0.99 a,b | 6.2 ± 0.31 a | 6.3 ± 0.56 a |
| P leaf, A/shoot | cm2/shoot | 3573 ± 327 a | 3221 ± 461 a,b | 2798 ± 395 b | 2597 ± 532 b |
| SVP | Unit | SC, Control | SC, Stress | DC, Control | DC, Stress |
|---|---|---|---|---|---|
| L shoot, FW | g/plant | 155 ± 76 a | 63 ± 14 b | 69 ± 22 b | 55 ± 30 b |
| L leaves, FW | g/plant | 570 ± 108 a | 238 ± 62 c | 424 ± 72 b | 323 ± 71 c |
| Total L leaf A | m2/plant | 2.47 ± 0.50 a | 1.22 ± 0.18 c | 1.9 ± 0.28 b | 1.51 ± 0.35 b,c |
| Total leaf A | m2/plant | 7.84 ± 0.54 a,b | 6.55 ± 1.17 b | 8.13 ± 0.52 a | 7.77 ± 0.71 a |
| A per leaf | cm2 | 245 ± 5.6 a | 232 ± 29 a | 193 ± 16 b | 187 ± 24 b |
| Leaf A/g fruit | cm2/g | 13.1 ± 1.6 a | 10.6 ± 1.3 a | 10.1 ± 2.7 a | 10.0 ± 2.4 a |
| Yield | Kg/plant | 6.05 ± 0.96 b | 6.18 ± 0.37 a,b | 8.33 ± 1.39 a | 7.94 ± 1.36 a,b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aru, V.; Nittnaus, A.P.; Sørensen, K.M.; Engelsen, S.B.; Toldam-Andersen, T.B. Effects of Water Stress, Defoliation and Crop Thinning on Vitis vinifera L. cv. Solaris: Part I: Plant Responses, Fruit Development and Fruit Quality. Metabolites 2022, 12, 363. https://doi.org/10.3390/metabo12040363
Aru V, Nittnaus AP, Sørensen KM, Engelsen SB, Toldam-Andersen TB. Effects of Water Stress, Defoliation and Crop Thinning on Vitis vinifera L. cv. Solaris: Part I: Plant Responses, Fruit Development and Fruit Quality. Metabolites. 2022; 12(4):363. https://doi.org/10.3390/metabo12040363
Chicago/Turabian StyleAru, Violetta, Andreas Paul Nittnaus, Klavs Martin Sørensen, Søren Balling Engelsen, and Torben Bo Toldam-Andersen. 2022. "Effects of Water Stress, Defoliation and Crop Thinning on Vitis vinifera L. cv. Solaris: Part I: Plant Responses, Fruit Development and Fruit Quality" Metabolites 12, no. 4: 363. https://doi.org/10.3390/metabo12040363
APA StyleAru, V., Nittnaus, A. P., Sørensen, K. M., Engelsen, S. B., & Toldam-Andersen, T. B. (2022). Effects of Water Stress, Defoliation and Crop Thinning on Vitis vinifera L. cv. Solaris: Part I: Plant Responses, Fruit Development and Fruit Quality. Metabolites, 12(4), 363. https://doi.org/10.3390/metabo12040363