Ammonia Concentration in the Eluent Influences Fragmentation Pattern of Triacylglycerols in Mass Spectrometry Analysis
Abstract
:1. Introduction
2. Results
2.1. Chromatographic Separation of Standards
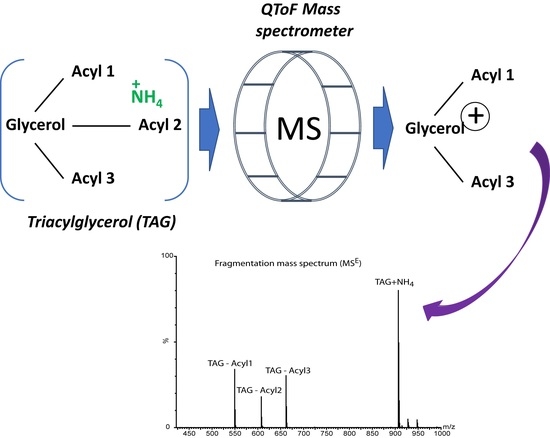
2.2. Mass Spectrometry and MSE Fragmentation Patterns
2.3. TAGs from Ovine Plasma
3. Discussion
3.1. UPLC-MS Method
3.2. Ammonium Concentration Effect on Fragmentation
3.3. Ovine Plasma TAGs
4. Materials and Methods
4.1. Reagents and Standards
4.2. Liquid Chromatography (UPLC)
4.3. Mass Spectrometry
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Decker, E.A. The Role of Stereospecific Saturated Fatty Acid Positions on Lipid Nutrition. Nutr. Rev. 2009, 54, 108–110. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, M.; Amate, L.; Gil, A. Absorption and Distribution of Dietary Fatty Acids from Different Sources. Early Hum. Dev. 2001, 65, S95–S101. [Google Scholar] [CrossRef]
- Björnson, E.; Östlund, Y.; Ståhlman, M.; Adiels, M.; Omerovic, E.; Jeppsson, A.; Borén, J.; Levin, M.C. Lipid Profiling of Human Diabetic Myocardium Reveals Differences in Triglyceride Fatty Acyl Chain Length and Degree of Saturation. Int. J. Cardiol. 2020, 320, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Guo, F.; Teng, F.; Ma, Y. A Novel Approach for the Reconstitution of Bovine Milk Fat Globules with Different-Melting-Temperature Triacylglycerol Cores. Food Chem. 2021, 345, 128563. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaga, K. Development of Analytical Methods and Nutritional Studies Using Synthetic Fatty Acids and Triacylglycerols. J. Oleo Sci. 2021, 70, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Ye, H. Overview of Lipidomic Analysis of Triglyceride Molecular Species in Biological Lipid Extracts. J. Agric. Food Chem. 2021, 69, 8895–8909. [Google Scholar] [CrossRef] [PubMed]
- Byrdwell, W.C.; Neff, W.E. Dual Parallel Electrospray Ionization and Atmospheric Pressure Chemical Ionization Mass Spectrometry (MS), MS/MS and MS/MS/MS for the Analysis of Triacylglycerols and Triacylglycerol Oxidation Products. Rapid Commun. Mass Spectrom. 2002, 16, 300–319. [Google Scholar] [CrossRef]
- Holčapek, M.; Dvořáková, H.; Lísa, M.; Girón, A.J.; Sandra, P.; Cvačka, J. Regioisomeric Analysis of Triacylglycerols Using Silver-Ion Liquid Chromatography–Atmospheric Pressure Chemical Ionization Mass Spectrometry: Comparison of Five Different Mass Analyzers. J. Chromatogr. A 2010, 1217, 8186–8194. [Google Scholar] [CrossRef]
- Abreu, S.; Heron, S.; Solgadi, A.; Joffre, F.; Tchapla, A.; Chaminade, P. Rapid Assessment of Triacylglycerol Fatty Acyls Composition by LC-APPI+-HRMS Using Monoacylglycerol like Fragments Intensities. Anal. Chim. Acta 2021, 1178, 338809. [Google Scholar] [CrossRef]
- Kalpio, M.; Linderborg, K.M.; Fabritius, M.; Kallio, H.; Yang, B. Strategy for Stereospecific Characterization of Natural Triacylglycerols Using Multidimensional Chromatography and Mass Spectrometry. J. Chromatogr. A 2021, 1641, 461992. [Google Scholar] [CrossRef]
- Li, X.; Evans, J.J. Examining the Collision-Induced Decomposition Spectra of Ammoniated Triglycerides as a Function of Fatty Acid Chain Length and Degree of Unsaturation. I. The OXO/YOY Series. Rapid Commun. Mass Spectrom. 2005, 19, 2528–2538. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Collins, E.J.; Evans, J.J. Examining the Collision-Induced Decomposition Spectra of Ammoniated Triglycerides as a Function of Fatty Acid Chain Length and Degree of Unsaturation. II. The PXP/YPY Series. Rapid Commun. Mass Spectrom. 2006, 20, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Balgoma, D.; Guitton, Y.; Evans, J.J.; Le Bizec, B.; Dervilly-Pinel, G.; Meynier, A. Modeling the Fragmentation Patterns of Triacylglycerides in Mass Spectrometry Allows the Quantification of the Regioisomers with a Minimal Number of Standards. Anal. Chim. Acta 2019, 1057, 60–69. [Google Scholar] [CrossRef]
- Makarov, P.; Zheng, D.; Le, D.; Evans, J.J. Impact of the Complexing Cation on the Sensitivity of Collision-Induced Dissociation Spectra to Fatty Acid Position for a Set of YXY/YYX-Type Triglycerides. Rapid Commun. Mass Spectrom. 2018, 32, 1591–1598. [Google Scholar] [CrossRef] [PubMed]
- Grossert, J.S.; Melanson, J.E.; Ramaley, L. Fragmentation Pathways of Cationized, Saturated, Short-Chain Triacylglycerols: Lithiated and Sodiated Tripropanoyl- and Trihexanoylglycerol. J. Am. Soc. Mass Spectrom. 2020, 31, 34–46. [Google Scholar] [CrossRef]
- Gutbrod, K.; Peisker, H.; Dörmann, P. Direct Infusion Mass for Complex. In Plant Lipids; Bartels, D., Dörmann, P., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2021; Volume 2295, pp. 101–115. ISBN 978-1-07-161361-0. [Google Scholar]
- Guan, M.; Dai, D.; Li, L.; Wei, J.; Yang, H.; Li, S.; Zhang, Y.; Lin, Y.; Xiong, S.; Zhao, Z. Comprehensive Qualification and Quantification of Triacylglycerols with Specific Fatty Acid Chain Composition in Horse Adipose Tissue, Human Plasma and Liver Tissue. Talanta 2017, 172, 206–214. [Google Scholar] [CrossRef]
- Liu, K.N.; Boxer, S.G. Target Membrane Cholesterol Modulates Single Influenza Virus Membrane Fusion Efficiency but Not Rate. Biophys. J. 2020, 118, 2426–2433. [Google Scholar] [CrossRef]
- Fabritius, M.; Linderborg, K.M.; Tarvainen, M.; Kalpio, M.; Zhang, Y.; Yang, B. Direct Inlet Negative Ion Chemical Ionization Tandem Mass Spectrometric Analysis of Triacylglycerol Regioisomers in Human Milk and Infant Formulas. Food Chem. 2020, 328, 126991. [Google Scholar] [CrossRef]
- George, A.D.; Gay, M.C.L.; Wlodek, M.E.; Trengove, R.D.; Murray, K.; Geddes, D.T. Untargeted Lipidomics Using Liquid Chromatography-Ion Mobility-Mass Spectrometry Reveals Novel Triacylglycerides in Human Milk. Sci. Rep. 2020, 10, 9255. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, W.; Tao, G.; Jin, Q.; Wang, X. Identification and Quantification of Triacylglycerols Using Ultraperformance Supercritical Fluid Chromatography and Quadrupole Time-of-Flight Mass Spectrometry: Comparison of Human Milk, Infant Formula, Other Mammalian Milk, and Plant Oil. J. Agric. Food Chem. 2021, 69, 8991–9003. [Google Scholar] [CrossRef]
- Masuda, K.; Abe, K.; Murano, Y. A Practical Method for Analysis of Triacylglycerol Isomers Using Supercritical Fluid Chromatography. J. Am. Oil Chem. Soc. 2021, 98, 21–29. [Google Scholar] [CrossRef]
- Arena, P.; Sciarrone, D.; Dugo, P.; Donato, P.; Mondello, L. Pattern-Type Separation of Triacylglycerols by Silver Thiolate×Non-Aqueous Reversed Phase Comprehensive Liquid Chromatography. Separations 2021, 8, 88. [Google Scholar] [CrossRef]
- Cabruja, M.; Priotti, J.; Domizi, P.; Papsdorf, K.; Kroetz, D.L.; Brunet, A.; Contrepois, K.; Snyder, M.P. In-Depth Triacylglycerol Profiling Using MS3 Q-Trap Mass Spectrometry. Anal. Chim. Acta 2021, 1184, 339023. [Google Scholar] [CrossRef]
- Bukowski, M.R.; Picklo, M.J. Simple, Rapid Lipidomic Analysis of Triacylglycerols in Bovine Milk by Infusion-Electrospray Mass Spectrometry. Lipids 2021, 56, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Leskinen, H.; Suomela, J.-P.; Kallio, H. Quantification of Triacylglycerol Regioisomers in Oils and Fat Using Different Mass Spectrometric and Liquid Chromatographic Methods. Rapid Commun. Mass Spectrom. 2007, 21, 2361–2373. [Google Scholar] [CrossRef]
- Xu, S.; Wei, F.; Xie, Y.; Lv, X.; Dong, X.; Chen, H. Research Advances Based on Mass Spectrometry for Profiling of Triacylglycerols in Oils and Fats and Their Applications. Electrophoresis 2018, 39, 1558–1568. [Google Scholar] [CrossRef] [PubMed]
- Hvattum, E. Analysis of Triacylglycerols with Non-Aqueous Reversed-Phase Liquid Chromatography and Positive Ion Electrospray Tandem Mass Spectrometry. Rapid Commun. Mass Spectrom. 2001, 15, 187–190. [Google Scholar] [CrossRef]
- Lísa, M.; Holčapek, M.; Sovová, H. Comparison of Various Types of Stationary Phases in Non-Aqueous Reversed-Phase High-Performance Liquid Chromatography–Mass Spectrometry of Glycerolipids in Blackcurrant Oil and Its Enzymatic Hydrolysis Mixture. J. Chromatogr. A 2009, 1216, 8371–8378. [Google Scholar] [CrossRef]
- Gazlay, W.; Evans, J.J. The Impact of the Complexing Agent on the Sensitivity of Collision-induced Dissociation Spectra to Fatty Acid Position for a Set of XYZ-type Triglycerides. Rapid Commun. Mass Spectrom. 2022, 36, e9226. [Google Scholar] [CrossRef]
- Hsu, F.-F.; Turk, J. Structural Characterization of Triacylglycerols as Lithiated Adducts by Electrospray Ionization Mass Spectrometry Using Low-Energy Collisionally Activated Dissociation on a Triple Stage Quadrupole Instrument. J. Am. Soc. Mass Spectrom. 1999, 10, 587–599. [Google Scholar] [CrossRef] [Green Version]
- Mamo, J.C.L.; Snoswell, A.M.; Topping, D.L. Plasma Triacylglycerol Secretion in Sheep. Biochim. Biophys. Acta BBA-Lipids Lipid Metab. 1983, 753, 272–275. [Google Scholar] [CrossRef]
- Fontecha, J.; Goudjil, H.; Ríos, J.J.; Fraga, M.J.; Juárez, M. Identity of the Major Triacylglycerols in Ovine Milk Fat. Int. Dairy J. 2005, 15, 1217–1224. [Google Scholar] [CrossRef] [Green Version]
- Cross, C.E.; Forte, T.M.; Gunther, R.A.; Kramer, G.C.; Lindgren, F.T.; Demling, R. Lipoprotein Profiles in Sheep Plasma and Lung Lymph. Chest 1983, 83, 93S–94S. [Google Scholar] [CrossRef] [PubMed]
- Khaki, Z.; Khazraiinia, P.; Chegini, S.; Khazraee Nia, S. Comparative Study of Serum Lipid Profile in Chicken, Ostrich, Cattle, and Sheep. Comp. Clin. Pathol. 2012, 21, 259–263. [Google Scholar] [CrossRef]
- Manis, C.; Addis, M.; Sitzia, M.; Scano, P.; Garau, V.; Cabiddu, A.; Caredda, M.; Pirisi, A.; Pulina, A.; Roggero, P.; et al. Untargeted Lipidomics of Ovine Milk to Analyse the Influence of Different Diet Regimens. J. Dairy Res. 2021, 88, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Plumb, R.S.; Johnson, K.A.; Rainville, P.; Smith, B.W.; Wilson, I.D.; Castro-Perez, J.M.; Nicholson, J.K. UPLC/MSE; a New Approach for Generating Molecular Fragment Information for Biomarker Structure Elucidation. Rapid Commun. Mass Spectrom. 2006, 20, 1989–1994. [Google Scholar] [CrossRef]













| Fragment m/z | Fragment Elemental Composition | Retention Time (min) | MSE Peak Intensity | TAG | TAG Elemental Composition | TAG MS Peak Intensity | Fatty Acyl Lost | Potential TAG | Calculated Fragment Intensity | |
|---|---|---|---|---|---|---|---|---|---|---|
| sn-1 or sn-3 position | sn-2 position | |||||||||
| (54:4) | ||||||||||
| 549.49 | C35H65O4 | 5.89 | 6190 | C57H102O6 | 15,800 | C22:3 | 22:3/-/- | 5830.2 | 2970.4 | |
| 599.51 | C39H67O4 | 6.12 | 2670 | C57H102O6 | 15,800 | C18:0 | 18:0/18:2/18:2 | 5830.2 | 2970.4 | |
| 603.53 | C39H71O4 | 5.94 | 11,000 | C57H102O6 | 15,800 | C18:2 | 18:2/18:0/18:2 | 5830.2 | 2970.4 | |
| (50:2) | ||||||||||
| 549.49 | C35H65O4 | 5.89 | 6190 | C53H98O6 | 3700 | C18:1 | 18:1/14:0/18:1 | 1365.3 | 695.6 | |
| 599.51 | C39H67O4 | 6.12 | 2670 | C53H98O6 | 3700 | C18:2 | 18:2/14:0/- | 1365.3 | 695.6 | |
| 603.53 | C39H71O4 | 5.94 | 11,000 | C53H98O6 | 3700 | C14:0 | 14:0/18:1/18:1 | 1365.3 | 695.6 | |
| m/z [M+ACN+NH4]+ | m/z [M+NH4]+ | Rt (min) 9.13 mM [NH4OH] | Elemental Composition [M+ACN+NH4]+ | TAG | Acyl Chains |
|---|---|---|---|---|---|
| 697.6086 | 656.5835 | 1.76 | C41H81N2O6 | TAG(36:0) | (12:0/12:0/12:0) |
| 723.6227 | 682.589 | 1.76 | C43H83N2O6 | TAG(38:1) | (12:0/12:0/14:1) |
| 749.6391 | 708.6163 | 1.78 | C45H85N2O6 | TAG(40:2) | (12:0/14:1/14:1) |
| 775.6596 | 734.6071 | 1.78 | C47H87N2O6 | TAG(42:3) | (14:1/14:1/14:1) Too low |
| 823.7106 | 782.6843 | 1.72 | C49H95N2O7 | Oxidized TAG? | TAG(44:1)(OH) |
| 849.7297 | 1.76 | C51H97N2O7 | Oxidized TAG? | TAG(46:2)(OH) | |
| 877.7574 | 1.95 | C53H101N2O7 | Oxidized TAG? | TAG(48:2)(OH) | |
| 725.6398 | 684.6135 | 2.09 | C43H85N2O6 | TAG(38:1) | (12:0/12:0/14:0) |
| 751.6572 | 710.6262 | 2.06 | C45H87N2O6 | TAG(40:1) | (12:0/12:0/16:1) |
| 777.6715 | 736.6446 | 2.06 | C47H89N2O6 | TAG(42:2) | (14:1/16:1/12:0) (14:1/14:1/14:0) |
| 803.688 | 762.6505 | 2.11 | C49H91N2O6 | TAG(44:3) | (14:1/14:1/16:1) |
| 753.6713 | 712.643 | 2.48 | C45H89N2O6 | TAG(40:0) | (12:0/12:0/16:0) |
| 779.6881 | 712.6439 | 2.42 | C47H91N2O6 | TAG(42:1) | (14:1/16:1/12:0) |
| 805.7037 | 764.6792 | 2.44 | C49H93N2O6 | TAG(44:2) | (14:1/16:1/14:0) (14:1/16:0/14:1) |
| 831.7203 | 790.6948 | 2.48 | C51H95N2O6 | TAG(46:3) | (18:1/12:0/16:2) |
| 857.7362 | 816.7054 | 2.49 | C53H97N2O6 | TAG(48:4) | (18:2/18:2/12:0) |
| 781.702 | 740.6749 | 2.98 | C47H93N2O6 | TAG(42:0) | (16:0/14:0/12:0) (18:0/12:0/12:0) (14:0/14:0/14:0) |
| 807.7178 | 766.6906 | 2.91 | C49H95N2O6 | TAG(44:1) | 16:1/14:0/14:0 |
| 833.7346 | 792.7086 | 2.93 | C51H97N2O6 | TAG(46:2) | (16:1/12:0/18:1) |
| 859.7489 | 818.7203 | 2.89 | C53H99N2O6 | TAG(48:3) | (18:1/12:0/18:2) |
| 885.7668 | 844.7361 | 3.06/3.25 | C55H101N2O6 | TAG(50:4) | (14:1/16:1/20:2) (16:2/14:0/20:2) |
| 809.7333 | 768.7072 | 3.62 | C49H97N2O6 | TAG(44:0) | (16:0/14:0/14:0) |
| 835.7502 | 794.7231 | 3.53 | C51H99N2O6 | TAG(46:1) | (18:1/16:0/12:0) |
| 861.7655 | 820.7371 | 3.46 | C53H101N2O6 | TAG(48:2) | (18:1/12:0/18:1) |
| 887.7794 | 846.7521 | 3.50 | C55H103N2O6 | TAG(50:3) | (18:1/16:1/16:1) |
| 913.7956 | 872.7681 | 3.64 | C57H105N2O6 | TAG(52:4) | (16:1/18:1/18:2) |
| 939.8111 | 898.7817 | 3.88 | C59H107N2O6 | TAG(54:5) | (18:2/18:2/18:1) |
| 965.8273 | 924.7947 | 3.84 | C61H109N2O6 | TAG(56:6) | (20:4/18:1/18:1) |
| 837.7642 | 796.7369 | 4.44 | C51H101N2O6 | TAG(46:0) | (16:0/14:0/16:0) |
| 863.7798 | 822.7529 | 4.33 | C53H103N2O6 | TAG(48:1) | (16:0/18:1/14:0) (18:1/16:0/14:0) |
| 889.7956 | 848.7697 | 4.27 | C55H105N2O6 | TAG(50:2) | (18:1/16:0/16:1) |
| 915.8111 | 874.7841 | 4.27 | C57H107N2O6 | TAG(52:3) | (18:1/16:1/18:1) |
| 941.8257 | 900.7987 | 4.19 | C59H109N2O6 | TAG(54:4) | (18:1/18:2/18:1) |
| 967.8471 | 926.8208 | 4.56 | C61H111N2O6 | TAG(56:5) | (20:2/18:2/18:1) |
| 993.8668 | 952.7939 | 4.53 | C63H113N2O6 | TAG(58:4) | (20:2/20:1/18:1) |
| 891.8107 | 850.7844 | 5.35 | C55H107N2O6 | TAG(50:1) | (18:1/16:0/16:0) |
| 917.8276 | 876.8007 | 5.19 | C57H109N2O6 | TAG(52:2) | (18:1/18:1/16:0) |
| 943.8428 | 902.8156 | 5.11 | C59H111N2O6 | TAG(54:3) | (18:1/18:1/18:1) |
| 969.8508 | 928.8232 | 5.11 | C61H113N2O6 | TAG(56:4) | (20:2/18:1/18:1) |
| 865.7928 | 824.7779 | 5.47 | C53H105N2O6 | TAG(48:0) | (18:0/16:0/14:0) (14:0/20:0/14:0) |
| 905.8237 | 864.8032 | 5.93 | C56H109N2O6 | TAG(51:1) | (18:1/17:0/16:0) |
| 931.8417 | 890.8013 | 5.84 | C58H111N2O6 | TAG(53:2) | (18:1/17:0/18:1) |
| 957.8604 | 916.8351 | 5.73 | C60H113N2O6 | TAG(55:3) | (19:1/17:1/19:1) |
| 893.8301 | 878.8172 | 6.78 | C55H109N2O6 | TAG(50:0) | (16:0/18:0/16:0) |
| 919.8469 | 878.8172 | 6.61 | C57H111N2O6 | TAG(52:1) | (18:1/18:0/16:0) |
| 945.8621 | 904.8362 | 6.55 | C59H113N2O6 | TAG(54:2) | (18:1/18:0/18:1) |
| 971.8732 | 930.8461 | 6.49 | C61H109N2O6 | TAG(56:3) | (20:1/18:1/18:1) |
| 907.8419 | 866.8082 | 7.48 | C56H111N2O6 | TAG(51:0) | (17:0/17:0/17:0) |
| 933.8631 | 892.8331 | 7.38 | C58H113N2O6 | TAG(53:1) | (18:1/17:0/18:0) |
| 959.8741 | 918.8602 | 7.25 | C60H115N2O6 | TAG(55:2) | (18:1/18:0/19:1) |
| 973.8895 | 932.8611 | 8.02 | C61H117N2O6 | TAG(56:2) | (18:1/18:0/20:1) |
| 947.8745 | 906.8404 | 8.19 | C59H115N2O6 | TAG(54:1) | (18:1/18:0/18:0) (20:1/16:0/18:0) |
| 921.8611 | 880.8317 | 8.42 | C57H113N2O6 | TAG(52:0) | (18:0/18:0/16:0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velasco, M.; Balgoma, D.; Montero, O. Ammonia Concentration in the Eluent Influences Fragmentation Pattern of Triacylglycerols in Mass Spectrometry Analysis. Metabolites 2022, 12, 452. https://doi.org/10.3390/metabo12050452
Velasco M, Balgoma D, Montero O. Ammonia Concentration in the Eluent Influences Fragmentation Pattern of Triacylglycerols in Mass Spectrometry Analysis. Metabolites. 2022; 12(5):452. https://doi.org/10.3390/metabo12050452
Chicago/Turabian StyleVelasco, Marta, David Balgoma, and Olimpio Montero. 2022. "Ammonia Concentration in the Eluent Influences Fragmentation Pattern of Triacylglycerols in Mass Spectrometry Analysis" Metabolites 12, no. 5: 452. https://doi.org/10.3390/metabo12050452
APA StyleVelasco, M., Balgoma, D., & Montero, O. (2022). Ammonia Concentration in the Eluent Influences Fragmentation Pattern of Triacylglycerols in Mass Spectrometry Analysis. Metabolites, 12(5), 452. https://doi.org/10.3390/metabo12050452