Glycolytic Plasticity of Metastatic Lung Cancer Captured by Noninvasive 18F-FDG PET/CT and Serum 1H-NMR Analysis: An Orthotopic Murine Model Study
Abstract
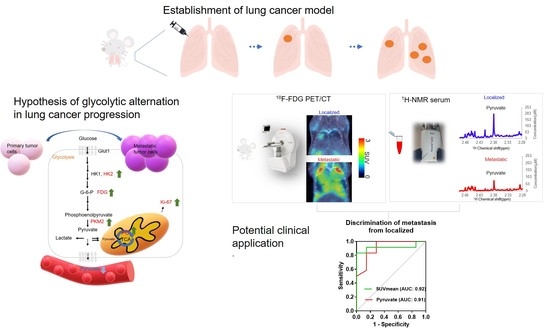
:1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. In Vivo and Ex Vivo Experimental Design
2.3. Surgical Procedure for Orthotopic Lung Cancer
2.4. In Vivo 18F-FDG PET/CT Imaging
2.5. Serum Extraction and 1H-NMR Metabolism Analysis
2.6. Ex Vivo Histopathological Assays of the Proliferation Rates and Glycolysis in Primary Tumors and Metastases
2.7. Statistical Analysis
3. Results
3.1. 18F-FDG PET/CT Detects Primary Tumors and Metastases
3.2. Metabolites in Serum by 1H-NMR Analysis
3.3. Elevated Proliferation Rates and Glycolysis in Metastatic Tumors
3.4. Outstanding AUC Values of 18F-FDG PET/CT Imaging Parameters and Serum Pyruvate Levels in ROC Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- 2022. Available online: https://www.cancer.org/cancer/lung-cancer/about/key-statistics.html (accessed on 13 May 2022).
- Lung Cancer-Non-Small Cell: Statistics. 2022. Available online: https://www.cancer.net/cancer-types/lung-cancer-non-small-cell/statistics (accessed on 11 October 2022).
- Gorad, S.S.; Ellingsen, C.; Bathen, T.F.; Mathiesen, B.S.; Moestue, S.A.; Rofstad, E.K. Identification of Metastasis-Associated Metabolic Profiles of Tumors by (1)H-HR-MAS-MRS. Neoplasia 2015, 17, 767–775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Q.; Qian, Y.; Yu, J.; Wong, C.C. Metabolic rewiring in the promotion of cancer metastasis: Mechanisms and therapeutic implications. Oncogene 2020, 39, 6139–6156. [Google Scholar] [CrossRef] [PubMed]
- Al-Jahdali, H.; Khan, A.N.; Loutfi, S.; Al-Harbi, A.S. Guidelines for the role of FDG-PET/CT in lung cancer management. J. Infect Public Health 2012, 5 (Suppl. S1), S35–S40. [Google Scholar] [CrossRef] [Green Version]
- Deppen, S.A.; Blume, J.D.; Kensinger, C.D.; Morgan, A.M.; Aldrich, M.C.; Massion, P.P.; Walker, R.C.; McPheeters, M.L.; Putnam, J.B., Jr.; Grogan, E.L. Accuracy of FDG-PET to diagnose lung cancer in areas with infectious lung disease: A meta-analysis. JAMA 2014, 312, 1227–1236. [Google Scholar] [CrossRef]
- Momcilovic, M.; Bailey, S.T.; Lee, J.T.; Zamilpa, C.; Jones, A.; Abdelhady, G.; Mansfield, J.; Francis, K.P.; Shackelford, D.B. Utilizing 18F-FDG PET/CT Imaging and Quantitative Histology to Measure Dynamic Changes in the Glucose Metabolism in Mouse Models of Lung Cancer. J. Vis. Exp. 2018, 137, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Weiss, I.D.; Ella, E.; Dominsky, O.; Smith, Y.; Abraham, M.; Wald, H.; Shlomai, Z.; Zamir, G.; Feigelson, S.W.; Shezen, E.; et al. In the hunt for therapeutic targets: Mimicking the growth, metastasis, and stromal associations of early-stage lung cancer using a novel orthotopic animal model. J. Thorac. Oncol. 2015, 10, 46–58. [Google Scholar] [CrossRef] [Green Version]
- Linden, H.M.; Krohn, K.A.; Livingston, R.B.; Mankoff, D.A. Monitoring targeted therapy: Is fluorodeoxylucose uptake a marker of early response? Clin. Cancer Res. 2006, 12, 5608–5610. [Google Scholar] [CrossRef] [Green Version]
- Beckonert, O.; Keun, H.C.; Ebbels, T.M.; Bundy, J.; Holmes, E.; Lindon, J.C.; Nicholson, J.K. Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nat. Protoc. 2007, 2, 2692–2703. [Google Scholar] [CrossRef]
- Thies, K.A.; Steck, S.; Knoblaugh, S.E.; Sizemore, S.T. Pathological Analysis of Lung Metastasis Following Lateral Tail-Vein Injection of Tumor Cells. J. Vis. Exp. 2020, 159, e61270. [Google Scholar] [CrossRef]
- Hu, J.M.; Sun, H.T. Serum proton NMR metabolomics analysis of human lung cancer following microwave ablation. Radiat. Oncol. 2018, 13, 40. [Google Scholar] [CrossRef]
- Pedersen, S.; Hansen, J.B.; Maltesen, R.G.; Szejniuk, W.M.; Andreassen, T.; Falkmer, U.; Kristensen, S.R. Identifying metabolic alterations in newly diagnosed small cell lung cancer patients. Metabol. Open 2021, 12, 100127. [Google Scholar] [CrossRef] [PubMed]
- Cusso, L.; Vaquero, J.J.; Bacharach, S.; Desco, M. Comparison of methods to reduce myocardial 18F-FDG uptake in mice: Calcium channel blockers versus high-fat diets. PLoS ONE 2014, 9, e107999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagana Gowda, G.A.; Raftery, D. Analysis of Plasma, Serum, and Whole Blood Metabolites Using (1)H NMR Spectroscopy. Methods Mol. Biol. 2019, 2037, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Perez, I.; Posma, J.M.; Serrano-Contreras, J.I.; Boulange, C.L.; Chan, Q.; Frost, G.; Stamler, J.; Elliott, P.; Lindon, J.C.; Holmes, E.; et al. Identifying unknown metabolites using NMR-based metabolic profiling techniques. Nat. Protoc. 2020, 15, 2538–2567. [Google Scholar] [CrossRef]
- Dona, A.C.; Jimenez, B.; Schafer, H.; Humpfer, E.; Spraul, M.; Lewis, M.R.; Pearce, J.T.; Holmes, E.; Lindon, J.C.; Nicholson, J.K. Precision high-throughput proton NMR spectroscopy of human urine, serum, and plasma for large-scale metabolic phenotyping. Anal. Chem 2014, 86, 9887–9894. [Google Scholar] [CrossRef]
- Richardson, P.M.; Parrott, A.J.; Semenova, O.; Nordon, A.; Duckett, S.B.; Halse, M.E. SABRE hyperpolarization enables high-sensitivity (1)H and (13)C benchtop NMR spectroscopy. Analyst 2018, 143, 3442–3450. [Google Scholar] [CrossRef] [Green Version]
- Chung, Y.H.; Tsai, C.K.; Yu, C.F.; Wang, W.L.; Yang, C.L.; Hong, J.H.; Yen, T.C.; Chen, F.H.; Lin, G. Radiation-Induced Metabolic Shifts in the Hepatic Parenchyma: Findings from (18)F-FDG PET Imaging and Tissue NMR Metabolomics in a Mouse Model for Hepatocellular Carcinoma. Molecules 2021, 26, 2573. [Google Scholar] [CrossRef]
- Bourafai-Aziez, A.; Jacob, D.; Charpentier, G.; Cassin, E.; Rousselot, G.; Moing, A.; Deborde, C. Development, Validation, and Use of (1)H-NMR Spectroscopy for Evaluating the Quality of Acerola-Based Food Supplements and Quantifying Ascorbic Acid. Molecules 2022, 27, 5614. [Google Scholar] [CrossRef]
- MetaboAnalyst 5.0. 2022. Available online: https://www.metaboanalyst.ca/ (accessed on 18 May 2022).
- Xia, J.; Psychogios, N.; Young, N.; Wishart, D.S. MetaboAnalyst: A web server for metabolomic data analysis and interpretation. Nucleic Acids Res. 2009, 37, W652–W660. [Google Scholar] [CrossRef] [Green Version]
- Walsby-Tickle, J.; Gannon, J.; Hvinden, I.; Bardella, C.; Abboud, M.I.; Nazeer, A.; Hauton, D.; Pires, E.; Cadoux-Hudson, T.; Schofield, C.J.; et al. Anion-exchange chromatography mass spectrometry provides extensive coverage of primary metabolic pathways revealing altered metabolism in IDH1 mutant cells. Commun Biol. 2020, 3, 247. [Google Scholar] [CrossRef]
- Chung, Y.H.; Yu, C.F.; Chiu, S.C.; Chiu, H.; Hsu, S.T.; Wu, C.R.; Yang, C.L.; Hong, J.H.; Yen, T.C.; Chen, F.H. Diffusion-weighted MRI and (18)F-FDG PET correlation with immunity in early radiotherapy response in BNL hepatocellular carcinoma mouse model: Timeline validation. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1733–1744. [Google Scholar] [CrossRef] [PubMed]
- Amoedo, N.D.; Rodrigues, M.F.; Pezzuto, P.; Galina, A.; da Costa, R.M.; de Almeida, F.C.; El-Bacha, T.; Rumjanek, F.D. Energy metabolism in H460 lung cancer cells: Effects of histone deacetylase inhibitors. PLoS ONE 2011, 6, e22264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiesel, V.A.; Sheeley, M.P.; Coleman, M.F.; Cotul, E.K.; Donkin, S.S.; Hursting, S.D.; Wendt, M.K.; Teegarden, D. Pyruvate carboxylase and cancer progression. Cancer Metab. 2021, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Kent, C. Regulatory enzymes of phosphatidylcholine biosynthesis: A personal perspective. Biochim. Biophys Acta 2005, 1733, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Balluff, B.; Arts, M.; Dubois, L.J.; van Loon, L.J.C.; Hackeng, T.M.; van Eijk, H.M.H.; Eijkel, G.; Heij, L.R.; Soons, Z.; et al. Mass spectrometry imaging of L-[ring-(13)C6]-labeled phenylalanine and tyrosine kinetics in non-small cell lung carcinoma. Cancer Metab. 2021, 9, 26. [Google Scholar] [CrossRef]
- Ghaddar, N.; Wang, S.; Michaud, V.; Kazimierczak, U.; Ah-Son, N.; Koromilas, A.E. Detection of Lung Tumor Progression in Mice by Ultrasound Imaging. J. Vis. Exp. 2020, 156, e60565. [Google Scholar] [CrossRef]
- Purandare, N.C.; Rangarajan, V. Imaging of lung cancer: Implications on staging and management. Indian J. Radiol. Imaging 2015, 25, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, S.; Inoue, H.; Ohba, S.; Kohda, Y.; Usami, I.; Masuda, T.; Kawada, M.; Nomoto, A. New metastatic model of human small-cell lung cancer by orthotopic transplantation in mice. Cancer Sci. 2015, 106, 367–374. [Google Scholar] [CrossRef] [Green Version]
- Greenspan, B.S. Role of PET/CT for precision medicine in lung cancer: Perspective of the Society of Nuclear Medicine and Molecular Imaging. Transl. Lung Cancer Res. 2017, 6, 617–620. [Google Scholar] [CrossRef] [Green Version]
- Spiro, J.E.; Rinneburger, M.; Hedderich, D.M.; Jokic, M.; Reinhardt, H.C.; Maintz, D.; Palmowski, M.; Persigehl, T. Monitoring treatment effects in lung cancer-bearing mice: Clinical CT and clinical MRI compared to micro-CT. Eur. Radiol. Exp. 2020, 4, 31. [Google Scholar] [CrossRef]
- Gupta, V.; Bamezai, R.N. Human pyruvate kinase M2: A multifunctional protein. Protein Sci. 2010, 19, 2031–2044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Zhang, S.; Liu, J.; Tian, Y.; Ma, B.; Xu, S.; Fu, Y.; Luo, Y. Secreted Pyruvate Kinase M2 Promotes Lung Cancer Metastasis through Activating the Integrin Beta1/FAK Signaling Pathway. Cell Rep. 2020, 30, 1780–1797.e1786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christen, S.; Lorendeau, D.; Schmieder, R.; Broekaert, D.; Metzger, K.; Veys, K.; Elia, I.; Buescher, J.M.; Orth, M.F.; Davidson, S.M.; et al. Breast Cancer-Derived Lung Metastases Show Increased Pyruvate Carboxylase-Dependent Anaplerosis. Cell Rep. 2016, 17, 837–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeBerardinis, R.J.; Lum, J.J.; Hatzivassiliou, G.; Thompson, C.B. The biology of cancer: Metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008, 7, 11–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, X.; Xiao, Z.; Hu, Y.; Zhang, X.; Fan, W. Combining (18)F-FDG PET/CT and Serum Lactate Dehydrogenase for Prognostic Evaluation of Small Cell Lung Cancer. Front. Pharmacol. 2020, 11, 592768. [Google Scholar] [CrossRef]
- Peng, L.; Du, B.; Cui, Y.; Luan, Q.; Li, Y.; Li, X. (18)F-FDG PET/CT for assessing heterogeneous metabolic response between primary tumor and metastases and prognosis in non-small cell lung cancer. Clin. Lung Cancer 2022, 23, 608–619. [Google Scholar] [CrossRef]
- Wumener, X.; Zhang, Y.; Wang, Z.; Zhang, M.; Zang, Z.; Huang, B.; Liu, M.; Huang, S.; Huang, Y.; Wang, P.; et al. Dynamic FDG-PET imaging for differentiating metastatic from non-metastatic lymph nodes of lung cancer. Front. Oncol. 2022, 12, 1005924. [Google Scholar] [CrossRef]
- Erdogan, Y.; Ozyurek, B.A.; Ozmen, O.; Yilmaz Demirci, N.; Duyar, S.S.; Dadali, Y.; Demirag, F.; Karakaya, J. The Evaluation of FDG PET/CT Scan Findings in Patients with Organizing Pneumonia Mimicking Lung Cancer. Mol. Imaging Radionucl. Ther. 2015, 24, 60–65. [Google Scholar] [CrossRef]







| Metabolites | VIP Score >1.5 | Fold Change (Metastasis/Localized) >1.2 or <0.8) | p-Value |
|---|---|---|---|
| Pyruvate | 2.3382 | 0.65992 | 0.004294 ** |
| O-phosphocholine | 1.8351 | 1.4604 | 0.057379 |
| Phenylalanine | 1.7429 | 0.75095 | 0.057379 |
| Creatine | 1.7341 | 0.79178 | 0.13494 |
| Fumarate | 1.5474 | 0.79517 | 0.34315 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, Y.-H.; Hung, T.-H.; Yu, C.-F.; Tsai, C.-K.; Weng, C.-C.; Jhang, F.; Chen, F.-H.; Lin, G. Glycolytic Plasticity of Metastatic Lung Cancer Captured by Noninvasive 18F-FDG PET/CT and Serum 1H-NMR Analysis: An Orthotopic Murine Model Study. Metabolites 2023, 13, 110. https://doi.org/10.3390/metabo13010110
Chung Y-H, Hung T-H, Yu C-F, Tsai C-K, Weng C-C, Jhang F, Chen F-H, Lin G. Glycolytic Plasticity of Metastatic Lung Cancer Captured by Noninvasive 18F-FDG PET/CT and Serum 1H-NMR Analysis: An Orthotopic Murine Model Study. Metabolites. 2023; 13(1):110. https://doi.org/10.3390/metabo13010110
Chicago/Turabian StyleChung, Yi-Hsiu, Tsai-Hsien Hung, Ching-Fang Yu, Cheng-Kun Tsai, Chi-Chang Weng, Fujie Jhang, Fang-Hsin Chen, and Gigin Lin. 2023. "Glycolytic Plasticity of Metastatic Lung Cancer Captured by Noninvasive 18F-FDG PET/CT and Serum 1H-NMR Analysis: An Orthotopic Murine Model Study" Metabolites 13, no. 1: 110. https://doi.org/10.3390/metabo13010110
APA StyleChung, Y. -H., Hung, T. -H., Yu, C. -F., Tsai, C. -K., Weng, C. -C., Jhang, F., Chen, F. -H., & Lin, G. (2023). Glycolytic Plasticity of Metastatic Lung Cancer Captured by Noninvasive 18F-FDG PET/CT and Serum 1H-NMR Analysis: An Orthotopic Murine Model Study. Metabolites, 13(1), 110. https://doi.org/10.3390/metabo13010110