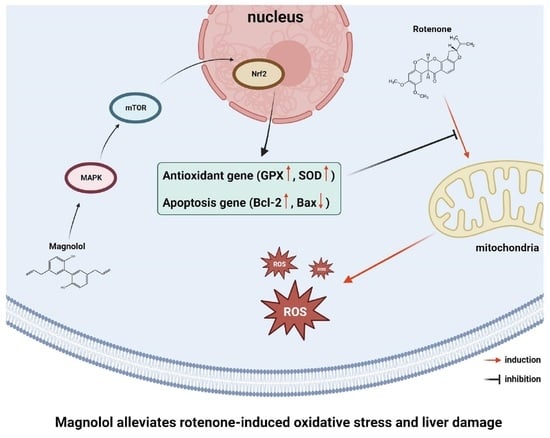
Magnolol as a Protective Antioxidant Alleviates Rotenone-Induced Oxidative Stress and Liver Damage through MAPK/mTOR/Nrf2 in Broilers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Sample Collection
2.3. Growth Performance Measurement
2.4. Oxidative Parameters Determination
2.5. Histopathological Examination
2.6. Apoptotic Status Analysis
2.7. qReal-Time PCR Analysis
2.8. Transcriptome Sequencing
2.9. Statistics Analysis
3. Results
3.1. Growth Performance
3.2. Antioxidant Index
3.3. Histopathological Observation
3.4. Relative Expression of Gene
3.5. RNA Sequencing
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mishra, B.; Jha, R. Oxidative Stress in the Poultry Gut: Potential Challenges and Interventions. Front. Vet. Sci. 2019, 6, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauridsen, C. From oxidative stress to inflammation: Redox balance and immune system. Poult. Sci. 2019, 98, 4240–4246. [Google Scholar] [CrossRef] [PubMed]
- Kpomasse, C.C.; Oke, O.E.; Houndonougbo, F.M.; Tona, K. Broiler production challenges in the tropics: A review. Vet. Med. Sci. 2021, 7, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Gazwi, H.; Mahmoud, M.E.; Toson, E. Author Correction: Analysis of the phytochemicals of Coriandrum sativum and Cicho-rium intybus aqueous extracts and their biological effects on broiler chickens. Sci. Rep. 2022, 12, 9964. [Google Scholar] [CrossRef] [PubMed]
- Fejercakova, A.; Vaskova, J.; Baca, M.; Vasko, L.; Marcincak, S.; Hertelyova, Z.; Petrasova, D.; Guothova, L. Effect of dietary microbially produced gamma-linolenic acid and plant extracts on enzymatic and non-enzymatic antioxidants in various broiler chicken organs. J. Anim. Physiol. Anim. Nutr. 2014, 98, 860–866. [Google Scholar] [CrossRef]
- Lee, D.; Szczepanski, M.; Lee, Y.J. Magnolol induces apoptosis via inhibiting the EGFR/PI3K/Akt signaling pathway in human prostate cancer cells. J. Cell. Biochem. 2009, 106, 1113–1122. [Google Scholar] [CrossRef]
- Lin, Q.; Zhao, J.; Xie, K.; Wang, Y.; Hu, G.; Jiang, G.; Dai, Q.; Fan, Z.; He, J.; He, X.; et al. Magnolol additive as a replacer of antibiotic enhances the growth performance of Linwu ducks. Anim. Nutr. 2017, 3, 132–138. [Google Scholar] [CrossRef]
- Cheng, J.; Dong, S.; Yi, L.; Geng, D.; Liu, Q. Magnolol abrogates chronic mild stress-induced depressive-like behaviors by inhibiting neuroinflammation and oxidative stress in the prefrontal cortex of mice. Int. Immunopharmacol. 2018, 59, 61–67. [Google Scholar] [CrossRef]
- Hou, W.; Yin, J.; Alimujiang, M.; Yu, X.; Ai, L.; Bao, Y.; Liu, F.; Jia, W. Inhibition of mitochondrial complex I improves glucose metabolism independently of AMPK activation. J. Cell. Mol. Med. 2018, 22, 1316–1328. [Google Scholar] [CrossRef] [Green Version]
- Balakrishnan, R.; Elangovan, N.; Mohankumar, T.; Nataraj, J.; Manivasagam, T.; Justin, T.A.; Essa, M.M.; Akbar, M.; Abdul, S.K.M. Isolongifolene attenuates rotenone-induced mitochondrial dysfunction, oxidative stress and apoptosis. Front. Biosci. 2018, 10, 248–261. [Google Scholar]
- Niu, Y.J.; Zhou, W.J.; Nie, Z.W.; Shin, K.T.; Cui, X.S. Melatonin enhances mitochondrial biogenesis and protects against rotenone-induced mitochondrial deficiency in early porcine embryos. J. Pineal. Res. 2020, 68, e12627. [Google Scholar] [CrossRef]
- Hua, H.; Ge, X.H.; Wu, M.Q.; Zhu, C.H.; Chen, L.H.; Yang, G.R.; Zhang, Y.; Huang, S.M.; Zhang, A.H.; Jia, Z.J. Rotenone Protects Against Acetaminophen-Induced Kidney Injury by Attenuating Oxidative Stress and Inflammation. Kidney Blood Press. Res. 2018, 43, 1297–1309. [Google Scholar] [CrossRef]
- Zhou, F.; Jiang, Z.; Yang, B.; Hu, Z. Magnolol exhibits anti-inflammatory and neuroprotective effects in a rat model of intracerebral haemorrhage. Brain Behav. Immun. 2019, 77, 161–167. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, H.; Du, E.; Jin, F.; Zheng, C.; Fan, Q.; Zhao, N.; Guo, W.; Zhang, W.; Huang, S.; et al. Effects of magnolol on egg production, egg quality, antioxidant capacity, and intestinal health of laying hens in the late phase of the laying cycle. Poult. Sci. 2021, 100, 835–843. [Google Scholar] [CrossRef]
- Xie, Q.; Xie, K.; Yi, J.; Song, Z.; Zhang, H.; He, X. The effects of magnolol supplementation on growth performance, meat quality, oxidative capacity, and intestinal microbiota in broilers. Poult. Sci. 2022, 101, 101722. [Google Scholar] [CrossRef]
- Akinmoladun, A.C.; Olaniyan, O.O.; Famusiwa, C.D.; Josiah, S.S.; Olaleye, M.T. Ameliorative effect of quercetin, catechin, and taxifolin on rotenone-induced testicular and splenic weight gain and oxidative stress in rats. J. Basic. Clin. Physiol. Pharmacol. 2020, 31, 37–46. [Google Scholar] [CrossRef]
- Ji, Y.; Gao, Y.; Chen, H.; Yin, Y.; Zhang, W. Indole-3-Acetic Acid Alleviates Nonalcoholic Fatty Liver Disease in Mice via Attenuation of Hepatic Lipogenesis, and Oxidative and Inflammatory Stress. Nutrients 2019, 11, 2062. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.H.; Lin, F.Y.; Liu, P.L.; Huang, Y.T.; Chiu, J.H.; Chang, Y.C.; Man, K.M.; Hong, C.Y.; Ho, Y.Y.; Lai, M.T. Antioxidative and hepatoprotective effects of magnolol on acetaminophen-induced liver damage in rats. Arch. Pharm. Res. 2009, 32, 221–228. [Google Scholar] [CrossRef]
- Subramaniyan, S.; Alugoju, P.; Sj, S.; Veerabhadrappa, B.; Dyavaiah, M. Magnolol protects Saccharomyces cerevisiae antioxidant-deficient mutants from oxidative stress and extends yeast chronological life span. FEMS. Microbiol. Lett. 2019, 366, fnz065. [Google Scholar] [CrossRef]
- Nishiyama, T.; Masuda, Y.; Izawa, T.; Ohnuma, T.; Ogura, K.; Hiratsuka, A. Magnolol protects PC12 cells from hydrogen peroxide or 6-hydroxydopamine induced cytotoxicity. J. Toxicol. Sci. 2019, 44, 753–758. [Google Scholar] [CrossRef] [Green Version]
- Trefts, E.; Gannon, M.; Wasserman, D.H. The liver. Curr. Biol. 2017, 27, R1147–R1151. [Google Scholar] [CrossRef] [PubMed]
- Alkatib, S.M.; Ismail, M.K.; AlMoula, A.H.; Alkennany, I.R. Hepatoprotective role of Legalon 70 against hydrogen peroxide in chickens. Int. J. Health Sci. 2019, 13, 17–21. [Google Scholar]
- Prior, N.; Inacio, P.; Huch, M. Liver organoids: From basic research to therapeutic applications. Gut 2019, 68, 2228–2237. [Google Scholar] [PubMed] [Green Version]
- Liu, X.; Hou, R.; Yan, J.; Xu, K.; Wu, X.; Lin, W.; Zheng, M.; Fu, J. Purification and characterization of Inonotus hispidus exopolysaccharide and its protective effect on acute alcoholic liver injury in mice. Int. J. Biol. Macromol. 2019, 129, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Samarghandian, S.; Azimi-Nezhad, M.; Farkhondeh, T.; Samini, F. Anti-oxidative effects of curcumin on immobilization-induced oxidative stress in rat brain, liver and kidney. Biomed. Pharmacother. 2017, 87, 223–229. [Google Scholar] [CrossRef]
- Kuo, N.C.; Huang, S.Y.; Yang, C.Y.; Shen, H.H.; Lee, Y.M. Involvement of HO-1 and Autophagy in the Protective Effect of Magnolol in Hepatic Steatosis-Induced NLRP3 Inflammasome Activation In Vivo and In Vitro. Antioxidants 2020, 9, 924. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.; Zhang, H.; Wang, T. Pterostilbene as a protective antioxidant attenuates diquat-induced liver injury and oxidative stress in 21-day-old broiler chickens. Poult. Sci. 2020, 99, 3158–3167. [Google Scholar] [CrossRef]
- Yeung, Y.T.; Aziz, F.; Guerrero-Castilla, A.; Arguelles, S. Signaling Pathways in Inflammation and Anti-inflammatory Therapies. Curr. Pharm. Des. 2018, 24, 1449–1484. [Google Scholar] [CrossRef]
- Condon, K.J.; Sabatini, D.M. Nutrient regulation of mTORC1 at a glance. J. Cell Sci. 2019, 132, jcs222570. [Google Scholar] [CrossRef]
- Chu, Q.; Yu, X.; Jia, R.; Wang, Y.; Zhang, Y.; Zhang, S.; Liu, Y.; Li, Y.; Chen, W.; Ye, X.; et al. Flavonoids fromApios americana Medikus Leaves Protect RAW264.7 Cells against Inflammation via Inhibition of MAPKs, Akt-mTOR Pathways, and Nfr2 Activation. Oxid. Med. Cell. Longev. 2019, 2019, 1563024. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Zhang, C.; Wang, Z.; Tang, Z.; Kuang, H.; Kong, A.N. Corynoline Isolated from Corydalis bungeana Turcz. Exhibits Anti-Inflammatory Effects via Modulation of Nfr2 and MAPKs. Molecules 2016, 21, 975. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.; Yang, J.; Yang, L.; Reis, F. Insights for Oxidative Stress and mTOR Signaling in Myocardial Ischemia/Reperfusion Injury under Diabetes. Oxid. Med. Cell. Longev. 2017, 2017, 6437412–6437467. [Google Scholar] [CrossRef]


| Ingredients | Content, % |
|---|---|
| Corn | 56.51 |
| Soybean meal | 35.30 |
| Soybean oil | 4.50 |
| Lysine (55%) | 0.35 |
| Methionine | 0.16 |
| Threonine | 0.06 |
| Nacl | 0.30 |
| Calcium hydrogen phosphate | 1.00 |
| Stone powder | 1.52 |
| Premix 1 | 0.30 |
| Total | 100.00 |
| Calculated nutrient levels | |
| Metabolizable energy (Kcal/kg) | 3039.94 |
| Crude protein, % | 20.65 |
| Calcium, % | 0.90 |
| Total phosphorus, % | 0.54 |
| Lysine, % | 1.27 |
| Methionine, % | 0.47 |
| Threonine, % | 0.83 |
| Gene 1 | Gene Bank ID | Primer Sequence, Sense/Antisense | Length |
|---|---|---|---|
| SOD | NM_205064.2 | TTGTCTGATGGAGATCATGGCTTC TGCTTGCCTTCAGGATTAAAGTGA | 98 |
| CAT | NM_001031215.1 | GTTGGCGGTAGGAGTCTGGTCT GTGGTCAAGGCATCTGGCTTCTG | 182 |
| GPX | NM_001163245.2 | CAAAGTTGCGGTCAGTGGA AGAGTCCCAGGCCTTTACTACTTTC | 136 |
| Nrf2 | NM_001396905.1 | GGGACGGTGACACAGGAACAAC GCTCTCCACAGCGGGAAATCAG | 97 |
| Keap1 | XM_015274015 | GCATCACAGCAGCGTGGAGAG GGCGTACAGCAGTCGGTTCAG | 118 |
| NQO1 | NM_001277620.2 | CCCGAGTGCTTTGTCTACGAGATG ATCAGGTCAGCCGCTTCAATCTTC | 107 |
| HO-1 | NM_205344.2 | GCTGGGAAGGAGAGTGAGAGGAC GCGACTGTGGTGGCGATGAAG | 107 |
| Bcl-2 | NM_205339.3 | GCTGCTTTACTCTTGGGGGT CTTCAGCACTATCTCGCGGT | 128 |
| Bax | XM_422067 | GGTGACAGGGATCGTCACAG TAGGCCAGGAACAGGGTGAAG | 108 |
| Caspase-3 | NM_204725.2 | TGGTGGAGGTGGAGGAGC TGTCTGTCATCATGGCTCTTG | 183 |
| XIAP | NM_204588.3 | ACCAAAAGAAAGCCCACT CATTCCTTACAAGCAACC | 147 |
| β-actin | NM_205518.2 | CTCTGACTGACCGCGTTACT TACCAACCATCACACCCTGAT | 172 |
| Items | CON | ROT | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| CON | MAG | CON | MAG | ANOVA 1 | Diet | Stress | D × S | |
| IBW (g) | 41.83 ± 0.35 | 41.71 ± 0.18 | 41.65 ± 0.30 | 41.97 ± 0.42 | 0.371 | 0.467 | 0.757 | 0.118 |
| 21 day BW (g) | 864.58 ± 27.48 | 847.92 ± 24.11 | 870.14 ± 26.01 | 865.98 ± 28.92 | 0.505 | 0.351 | 0.291 | 0.573 |
| 1–21 day ADFI (g) | 46.18 ± 1.33 | 46.49 ± 2.58 | 47.21 ± 2.02 | 46.86 ± 2.70 | 0.864 | 0.980 | 0.448 | 0.718 |
| 1–21 day ADG (g) | 39.18 ± 1.30 | 38.39 ± 1.15 | 39.45 ± 1.23 | 39.24 ± 1.37 | 0.503 | 0.344 | 0.291 | 0.582 |
| 1–21 day F/G | 1.18 ± 0.03 | 1.21 ± 0.09 | 1.20 ± 0.03 | 1.19 ± 0.06 | 0.765 | 0.553 | 0.972 | 0.385 |
| FBW (g) | 1463.70 ± 49.50 a | 1464.96 ± 36.48 a | 1342.85 ± 94.09 b | 1414.94 ± 79.10 ab | 0.019 | 0.206 | 0.006 | 0.221 |
| ADFI post injection (g) | 111.08 ± 7.23 | 113.50 ± 3.23 | 101.16 ± 14.03 | 121.88 ± 36.18 | 0.367 | 0.168 | 0.925 | 0.271 |
| ADG during injection (g) | 74.89 ± 4.11 ab | 77.13 ± 4.43 a | 59.09 ± 8.85 c | 68.62 ± 6.98 b | <0.001 | 0.035 | <0.001 | 0.178 |
| Items | CON | ROT | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| CON | MAG | CON | MAG | ANOVA 1 | Diet | Stress | D × S | |
| Serum | ||||||||
| T-AOC (U/mL) | 0.10 ± 0.02 ab | 0.11 ± 0.02 a | 0.09 ± 0.02 b | 0.09 ± 0.01 ab | 0.040 | 0.149 | 0.023 | 0.633 |
| CAT (U/mL) | 106.54 ± 2.97 a | 104.18 ± 2.87 a | 100.61 ± 3.90 b | 103.56 ± 5.51 ab | 0.007 | 0.793 | 0.006 | 0.025 |
| GSH (μmol/L) | 4.03 ± 0.84 b | 4.62 ± 0.42 a | 3.03 ± 0.49 c | 3.89 ± 0.66 b | <0.001 | 0.001 | <0.001 | 0.508 |
| GSH-PX (U/mL) | 2178.84 ± 165.91 ab | 2265.22 ± 149.00 a | 1803.58 ± 210.47 c | 2020.65 ± 234.57 b | <0.001 | 0.255 | <0.001 | 0.010 |
| T-SOD (U/mL) | 224.41 ± 38.63 | 254.39 ± 43.91 | 209.03 ± 43.78 | 211.63 ± 35.15 | 0.123 | 0.254 | 0.047 | 0.336 |
| MDA (nmol/mL) | 4.67 ± 0.94 b | 5.22 ± 0.82 ab | 5.67 ± 1.14 a | 4.49 ± 0.89 b | 0.025 | 0.276 | 0.638 | 0.005 |
| 8-OHdG (ng/mL) | 6.91 ± 0.51 | 6.87 ± 0.27 | 7.34 ± 0.37 | 7.22 ± 0.40 | 0.061 | 0.534 | 0.009 | 0.777 |
| AST(U/L) | 49.52 ± 5.16 b | 51.00 ± 7.97 b | 61.15 ± 10.88 a | 53.35 ± 3.25 ab | 0.030 | 0.263 | 0.018 | 0.106 |
| Liver | ||||||||
| T-AOC (U/mgprot) | 0.023 ± 0.00 a | 0.022 ± 0.00 a | 0.016 ± 0.00 b | 0.018 ± 0.00 b | <0.001 | 0.928 | <0.001 | 0.031 |
| CAT (U/mgprot) | 95.50 ± 15.20 a | 80.39 ± 23.02 a | 50.04 ± 8.03 b | 51.63 ± 12.02 b | <0.001 | 0.224 | <0.001 | 0.135 |
| GSH (μmol/gprot) | 6.19 ± 0.76 | 6.32 ± 0.98 | 5.60 ± 0.95 | 5.99 ± 0.69 | 0.470 | 0.432 | 0.167 | 0.685 |
| GSH-PX (U/mgprot) | 61.87 ± 5.21 a | 54.46 ± 5.99 b | 42.15 ± 2.35 d | 46.93 ± 6.38 c | <0.001 | 0.406 | <0.001 | <0.001 |
| T-SOD (U/mgprot) | 39.23 ± 3.89 a | 38.04 ± 4.94 a | 31.99 ± 2.84 b | 33.00 ± 3.48 b | <0.001 | 0.939 | <0.001 | 0.338 |
| MDA (nmol/mgprot) | 0.61 ± 0.10 | 0.57 ± 0.10 | 0.67 ± 0.10 | 0.65 ± 0.06 | 0.141 | 0.368 | 0.034 | 0.812 |
| 8-OHdG (ng/mL) | 0.78 ± 0.11 b | 0.76 ± 0.07 b | 0.89 ± 0.12 a | 0.81 ± 0.09 a | 0.026 | 0.108 | 0.018 | 0.341 |
| AST(U/gprot) | 46.27 ± 9.30 c | 50.58 ± 10.95 bc | 67.06 ± 14.71 a | 61.82 ± 14.44 ab | 0.004 | 0.912 | 0.001 | 0.266 |
| Items | CON | ROT | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| CON | MAG | CON | MAG | ANOVA 1 | Diet | Stress | D × S | |
| CAT | 1.00 ± 0.40 | 0.89 ± 0.33 | 0.92 ± 0.48 | 0.55 ± 0.28 | 0.101 | 0.084 | 0.140 | 0.351 |
| GPX | 1.00 ± 0.49 c | 1.56 ± 0.52 b | 0.69 ± 0.32 bc | 2.77 ± 0.61 a | <0.001 | <0.001 | 0.043 | 0.002 |
| SOD | 1.00 ± 0.58 b | 1.82 ± 0.74 ab | 2.13 ± 1.15 a | 2.77 ± 0.92 a | 0.020 | 0.052 | 0.008 | 0.803 |
| Nrf2 | 1.00 ± 0.48 b | 0.85 ± 0.37 b | 1.61 ± 0.32 a | 1.70 ± 0.69 a | 0.005 | 0.864 | 0.001 | 0.508 |
| Keap1 | 1.00 ± 0.51 b | 1.04 ± 0.12 b | 1.82 ± 0.68 a | 1.88 ± 0.44 a | 0.007 | 0.829 | 0.001 | 0.963 |
| HO-1 | 1.00 ± 0.51 b | 1.26 ± 0.40 ab | 1.79 ± 0.66 a | 1.18 ± 0.38 b | 0.073 | 0.371 | 0.077 | 0.037 |
| NQO1 | 1.00 ± 0.52 | 0.91 ± 0.32 | 0.68 ± 0.29 | 1.00 ± 0.61 | 0.554 | 0.537 | 0.528 | 0.278 |
| Bcl-2 | 1.00 ± 0.25 bc | 1.44 ± 0.30 a | 0.80 ± 0.22 c | 1.34 ± 0.53 ab | 0.009 | 0.001 | 0.227 | 0.706 |
| Bax | 1.00 ± 0.25 bc | 1.39 ± 0.22 a | 1.49 ± 0.15 a | 0.98 ± 0.28 b | 0.010 | 0.466 | 0.619 | <0.001 |
| Caspase-3 | 1.00 ± 0.25 | 0.91 ± 0.16 | 1.43 ± 0.31 | 1.14 ± 0.23 | 0.217 | 0.049 | 0.001 | 0.282 |
| XIAP | 1.00 ± 0.25 | 0.87 ± 0.20 | 2.57 ± 0.52 | 1.70 ± 0.45 | 0.178 | 0.003 | <0.001 | 0.022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, W.; Zhou, N.; Song, Z.; Zhang, H.; He, X. Magnolol as a Protective Antioxidant Alleviates Rotenone-Induced Oxidative Stress and Liver Damage through MAPK/mTOR/Nrf2 in Broilers. Metabolites 2023, 13, 84. https://doi.org/10.3390/metabo13010084
Peng W, Zhou N, Song Z, Zhang H, He X. Magnolol as a Protective Antioxidant Alleviates Rotenone-Induced Oxidative Stress and Liver Damage through MAPK/mTOR/Nrf2 in Broilers. Metabolites. 2023; 13(1):84. https://doi.org/10.3390/metabo13010084
Chicago/Turabian StylePeng, Weishi, Nanxuan Zhou, Zehe Song, Haihan Zhang, and Xi He. 2023. "Magnolol as a Protective Antioxidant Alleviates Rotenone-Induced Oxidative Stress and Liver Damage through MAPK/mTOR/Nrf2 in Broilers" Metabolites 13, no. 1: 84. https://doi.org/10.3390/metabo13010084
APA StylePeng, W., Zhou, N., Song, Z., Zhang, H., & He, X. (2023). Magnolol as a Protective Antioxidant Alleviates Rotenone-Induced Oxidative Stress and Liver Damage through MAPK/mTOR/Nrf2 in Broilers. Metabolites, 13(1), 84. https://doi.org/10.3390/metabo13010084