Sex-Specific Relationships between HDL-Cholesterol Levels and 10-Year Mortality in Individuals with Atherosclerotic Cardiovascular Disease: A Nationwide Cohort Study of South Koreans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources
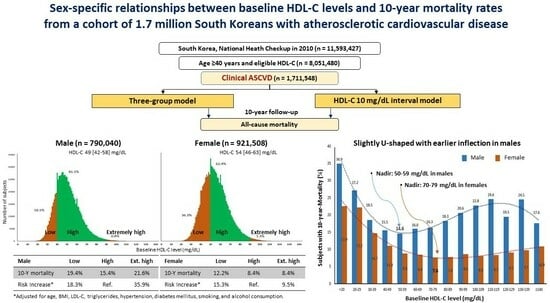
2.2. Study Design and Subject Selection
2.3. Definitions and Models
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Sex-Stratified Three-Group Comparisons of 10-Year All-Cause Mortality
3.3. Hazard Ratios for 10-Year All-Cause Mortality
3.4. Distribution of 10-Year Mortality Stratified by Baseline HDL-C Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ko, D.T.; Alter, D.A.; Guo, H.; Koh, M.; Lau, G.; Austin, P.C.; Booth, G.L.; Hogg, W.; Jackevicius, C.A.; Lee, D.S.; et al. High-Density Lipoprotein Cholesterol and Cause-Specific Mortality in Individuals without Previous Cardiovascular Conditions: The CANHEART Study. J. Am. Coll. Cardiol. 2016, 68, 2073–2083. [Google Scholar] [CrossRef]
- Madsen, C.M.; Varbo, A.; Nordestgaard, B.G. Extreme high high-density lipoprotein cholesterol is paradoxically associated with high mortality in men and women: Two prospective cohort studies. Eur. Heart J. 2017, 38, 2478–2486. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.W.; Park, S.J.; Yi, J.J.; Ohrr, H.; Kim, H. High-density lipoprotein cholesterol and all-cause mortality by sex and age: A prospective cohort study among 15.8 million adults. Int. J. Epidemiol. 2021, 50, 902–913. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.S.; Jeong, H.J.; Kim, H.; Hwang, H.K.; Hur, M.; Lee, S. Sex-Specific U-Shaped Relationships Between High-Density Lipoprotein Cholesterol Levels and 10-year Major Adverse Cardiovascular Events: A Nationwide Cohort Study of 5.7 Million South Koreans. Ann. Lab. Med. 2022, 42, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Wolska, A.; Remaley, A.T. Measuring LDL-cholesterol: What is the best way to do it? Curr. Opin. Cardiol. 2020, 35, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.H.; Lee, W.Y.; Kim, S.S.; Kang, J.H.; Kang, J.H.; Kim, K.K.; Kim, B.Y.; Kim, Y.H.; Kim, W.J.; Kim, E.M.; et al. 2018 Korean Society for the Study of Obesity Guideline for the Management of Obesity in Korea. J. Obes. Metab. Syndr. 2019, 28, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Kaur, M.; Ahuja, K.R.; Khubber, S.; Zhou, L.; Verma, B.R.; Meenakshisundaram, C.; Gad, M.M.; Saad, A.; Dhaliwal, K.; Isogai, T.; et al. Effect of High-Density Lipoprotein Cholesterol Levels on Overall Survival and Major Adverse Cardiovascular and Cerebrovascular Events. Am. J. Cardiol. 2021, 146, 8–14. [Google Scholar] [CrossRef]
- Liu, C.; Dhindsa, D.; Almuwaqqat, Z.; Ko, Y.A.; Mehta, A.; Alkhoder, A.A.; Alras, Z.; Desai, S.R.; Patel, K.J.; Hooda, A.; et al. Association Between High-Density Lipoprotein Cholesterol Levels and Adverse Cardiovascular Outcomes in High-risk Populations. JAMA Cardiol. 2022, 7, 672–680. [Google Scholar] [CrossRef]
- Yang, H.S.; Hur, M.; Lee, S. Gender-Specific Cutoffs for Very High High-Density Lipoprotein Cholesterol Levels May Impact the Study Results. Am. J. Cardiol. 2023, 188, 120. [Google Scholar] [CrossRef]
- Liu, C.; Dhindsa, D.; Almuwaqqat, Z.; Sun, Y.V.; Quyyumi, A.A. Very High High-Density Lipoprotein Cholesterol Levels and Cardiovascular Mortality. Am. J. Cardiol. 2023, 188, 120–121. [Google Scholar] [CrossRef] [PubMed]
- Kappert, K.; Böhm, M.; Schmieder, R.; Schumacher, H.; Teo, K.; Yusuf, S.; Sleight, P.; Unger, T. Impact of sex on cardiovascular outcome in patients at high cardiovascular risk: Analysis of the Telmisartan Randomized Assessment Study in ACE-Intolerant Subjects With Cardiovascular Disease (TRANSCEND) and the Ongoing Telmisartan Alone and in Combination With Ramipril Global End Point Trial (ONTARGET). Circulation 2012, 126, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Kashani, K.; Rosner, M.H.; Ostermann, M. Creatinine: From physiology to clinical application. Eur. J. Intern. Med. 2020, 72, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Holven, K.B.; Roeters van Lennep, J. Sex differences in lipids: A life course approach. Atherosclerosis 2023, 384, 117270. [Google Scholar] [CrossRef]
- Williams, C.M. Lipid metabolism in women. Proc. Nutr. Soc. 2004, 63, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Johannesen, C.D.L.; Mortensen, M.B.; Langsted, A.; Nordestgaard, B.G. Apolipoprotein B and Non-HDL Cholesterol Better Reflect Residual Risk Than LDL Cholesterol in Statin-Treated Patients. J. Am. Coll. Cardiol. 2021, 77, 1439–1450. [Google Scholar] [CrossRef]
- Kawamoto, R.; Kikuchi, A.; Akase, T.; Ninomiya, D.; Kumagi, T. Low density lipoprotein cholesterol and all-cause mortality rate: Findings from a study on Japanese community-dwelling persons. Lipids Health Dis. 2021, 20, 105. [Google Scholar] [CrossRef]
- Zhou, L.; Wu, Y.; Yu, S.; Shen, Y.; Ke, C. Low-density lipoprotein cholesterol and all-cause mortality: Findings from the China health and retirement longitudinal study. BMJ Open 2020, 10, e036976. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Zheng, L.; Hazen, S.L. Formation of dysfunctional high-density lipoprotein by myeloperoxidase. Trends Cardiovasc. Med. 2005, 15, 212–219. [Google Scholar] [CrossRef]
- Soria-Florido, M.T.; Castañer, O.; Lassale, C.; Estruch, R.; Salas-Salvadó, J.; Martínez-González, M.; Corella, D.; Ros, E.; Arós, F.; Elosua, R.; et al. Dysfunctional High-Density Lipoproteins Are Associated With a Greater Incidence of Acute Coronary Syndrome in a Population at High Cardiovascular Risk: A Nested Case-Control Study. Circulation 2020, 141, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Martagon, A.J.; Zubirán, R.; González-Arellanes, R.; Praget-Bracamontes, S.; Rivera-Alcántara, J.A.; Aguilar-Salinas, C.A. HDL abnormalities in type 2 diabetes: Clinical implications. Atherosclerosis 2023, 117213. [Google Scholar] [CrossRef] [PubMed]
- Agarwala, A.P.; Rodrigues, A.; Risman, M.; McCoy, M.; Trindade, K.; Qu, L.; Cuchel, M.; Billheimer, J.; Rader, D.J. High-Density Lipoprotein (HDL) Phospholipid Content and Cholesterol Efflux Capacity Are Reduced in Patients With Very High HDL Cholesterol and Coronary Disease. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1515–1519. [Google Scholar] [CrossRef] [PubMed]
- von Eckardstein, A.; Nordestgaard, B.G.; Remaley, A.T.; Catapano, A.L. High-density lipoprotein revisited: Biological functions and clinical relevance. Eur. Heart J. 2023, 44, 1394–1407. [Google Scholar] [CrossRef]
- Barzilay, J.I.; Buzkova, P.; Kuller, L.H.; Cauley, J.A.; Fink, H.A.; Sheets, K.; Robbins, J.A.; Carbone, L.D.; Elam, R.E.; Mukamal, K.J. The Association of Lipids and Lipoproteins with Hip Fracture Risk: The Cardiovascular Health Study. Am. J. Med. 2022, 135, 1101–1108.e1. [Google Scholar] [CrossRef]
- Goff, D.C., Jr.; Lloyd-Jones, D.M.; Bennett, G.; Coady, S.; D’Agostino, R.B., Sr.; Gibbons, R.; Greenland, P.; Lackland, D.T.; Levy, D.; O’Donnell, C.J.; et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 63, 2935–2959. [Google Scholar] [CrossRef]
- ASCVD Risk Estimator Plus: ACC Recommendation. Available online: https://tools.acc.org/ascvd-risk-estimator-plus/#!/calculate/estimate/ (accessed on 1 November 2023).
- Ganjali, S.; Hosseini, S.; Rizzo, M.; Kontush, A.; Sahebkar, A. Capacity of HDL to Efflux Cellular Cholesterol from Lipid-Loaded Macrophages Is Reduced in Patients with Familial Hypercholesterolemia. Metabolites 2023, 13, 197. [Google Scholar] [CrossRef]
- Yang, H.S.; Hur, M.; Kim, H.; Kim, S.J.; Shin, S.; Di Somma, S. HDL Subclass Analysis in Predicting Metabolic Syndrome in Koreans With High HDL Cholesterol Levels. Ann. Lab. Med. 2020, 40, 297–305. [Google Scholar] [CrossRef]



| Total | Male | Female | p Value | |
|---|---|---|---|---|
| Number of subjects, n (% of total cohort) | 1,711,548 (100) | 790,040 (46.2) | 921,508 (53.8) | _ |
| Age, years | 61.4 ± 10.3 | 60.7 ± 10.3 | 62.1 ± 10.3 | <0.0001 |
| 40–49 years, n (%) | 227,898 (13.3) | 122,244 (15.5) | 105,654 (11.5) | <0.0001 |
| 50–59 years, n (%) | 521,167 (30.5) | 246,790 (31.2) | 274,377 (29.3) | |
| 60–69 years, n (%) | 533,907 (31.2) | 242,738 (30.7) | 291,169 (31.6) | |
| 70–79 years, n (%) | 361,140 (21.1) | 151,911 (19.2) | 209,229 (22.7) | |
| ≥80 years, n (%) | 67,436 (3.9) | 26,357 (3.3) | 41,079 (4.5) | |
| BMI, kg/m2 | 24.47 ± 3.79 | 24.46 ± 2.97 | 24.48 ± 4.36 | <0.0001 |
| Obesity, n (%) | 691,695 (40.4) | 322,504 (40.8) | 369,191 (40.1) | <0.0001 |
| Abd. obesity, n (%) | 540,209 (31.6) | 247,495 (31.3) | 292,714 (31.8) | <0.0001 |
| Smoking, n (%) | ||||
| Never | 1,164,024 (68.2) | 278,817 (35.4) | 885,207 (96.3) | <0.0001 |
| Past | 291,584 (17.1) | 271,184 (35.7) | 10,400 (1.1) | |
| Current | 252,169 (14.8) | 228,484 (29.0) | 23,685 (2.6) | |
| Alcohol, n (%) | ||||
| None | 1,163,813 (68.2) | 355,762 (45.1) | 808,051 (87.9) | <0.0001 |
| 1 time/week | 205,152 (12.0) | 138,401 (17.6) | 66,751 (7.3) | |
| 2 times/week | 135,159 (7.9) | 112,196 (14.2) | 22,963 (2.5) | |
| ≥3 times/week | 203,297 (11.9) | 181,860 (23.1) | 21,437 (2.3) | |
| Systolic BP, mmHg | 127.3 ± 15.7 | 128.0 ± 15.1 | 126.7 ± 16.1 | <0.0001 |
| Diastolic BP, mmHg | 78.1 ± 10.0 | 78.9 ± 9.9 | 77.4 ± 10.0 | <0.0001 |
| Hypertension, n (%) | 1,044,507 (61.0) | 497,307 (63.0) | 547,200 (59.4) | <0.0001 |
| DM, n (%) | 399,287 (23.3) | 210,225 (26.6) | 189,062 (20.5) | <0.0001 |
| FBS, mg/dL | 98 [89–110] | 99 [90–114] | 96 [88–107] | <0.0001 |
| Blood test at baseline | ||||
| sCr, mg/dL | 0.9 [0.8–1.0] | 1.0 [0.9–1.1] | 0.8 [0.7–0.9] | <0.0001 |
| TC, mg/dL | 194 [168–220] | 187 [162–212] | 199 [174–226] | <0.0001 |
| LDL-C, mg/dL | 112 [89–137] | 107 [84–130] | 117 [94–142] | <0.0001 |
| HDL-C, mg/dL | 51 [44–61] | 49 [42–58] | 54 [46–63] | <0.0001 |
| Triglycerides, mg/dL | 122 [86–175] | 128 [90–186] | 117 [84–166] | <0.0001 |
| Non-HDL-C, mg/dL | 140 [116–167] | 136 [112–162] | 144 [120–171] | <0.0001 |
| Sex | Male | Female | ||||||
|---|---|---|---|---|---|---|---|---|
| Groups | Low (N = 142,893) | High (N = 640,800) | Extremely High (N = 6347) | p Value | Low (N = 334,453) | High (N = 574,941) | Extremely High (N = 12,114) | p Value |
| Age, years | 61.3 ±10.4 | 60.5 ±10.3 | 61.5 ±10.1 | <0.0001 † | 63.7 ±10.2 | 61.3 ±10.2 | 59.7 ±10.4 | <0.0001 * |
| 40–49 years, n (%) | 20,436 (14.3) | 101,005 (15.8) | 803 (12.7) | <0.0001 * | 29,538 (8.8) | 74,137 (12.9) | 1979 (16.3) | <0.0001 * |
| 50–59 years, n (%) | 43,114 (30.2) | 201,757 (31.5) | 1919 (30.2) | 86,503 (25.9) | 183,488 (31.9) | 4386 (36.2) | ||
| 60–69 years, n (%) | 43,616 (30.5) | 197,005 (30.7) | 2117 (33.4) | 110,614 (33.1) | 177,331 (30.8) | 3224 (26.6) | ||
| 70–79 years, n (%) | 30,185 (21.1) | 120,434 (18.8) | 1292 (20.4) | 88,328 (26.4) | 118,803 (20.7) | 2098 (17.3) | ||
| ≥80 years, n (%) | 5542 (3.9) | 20,599 (3.2) | 216 (3.4) | 19,470 (5.8) | 21,182 (3.7) | 427 (3.5) | ||
| BMI, kg/m2 | 25.1 ±2.9 | 24.3 ±3.0 | 22.8 ±3.1 | <0.0001 * | 24.8 ±5.8 | 24.3 ±3.3 | 23.4 ±3.4 | <0.0001 * |
| Obesity, n (%) | 70,045 (49.1) | 251,065 (39.2) | 1394 (22.0) | <0.0001 * | 148,502 (44.5) | 217,241 (37.8) | 3448 (28.5) | <0.0001 * |
| Abd. obesity, n (%) | 56,497 (39.6) | 189,853 (29.6) | 1145 (18.0) | <0.0001 * | 123,811 (37.1) | 166,292 (28.9) | 2611 (21.6) | <0.0001 * |
| Smoking, n (%) | ||||||||
| Never | 49,534 (34.7) | 227,058 (35.5) | 2225 (35.1) | <0.0001 † | 320,843 (96.1) | 552,874 (96.4) | 11,490 (95.0) | <0.0001 * |
| Past | 48,475 (34.0) | 230,583 (36.1) | 2126 (33.5) | 3732 (1.1) | 6476 (1.1) | 192 (1.6) | ||
| Current | 44,583 (31.3) | 181,912 (28.4) | 1989 (31.4) | 9496 (2.8) | 14,081 (2.5) | 408 (3.4) | ||
| Alcohol, n (%) | ||||||||
| None | 81,074 (56.9) | 273,028 (42.7) | 1660 (26.3) | <0.0001 * | 305,653 (91.6) | 493,298 (86.0) | 9100 (75.5) | <0.0001 * |
| 1 time/week | 25,139 (17.6) | 112,481 (17.6) | 781 (12.4) | 18,470 (5.5) | 46,960 (8.2) | 1321 (11.0) | ||
| 2 times/week | 15,893 (11.2) | 95,385 (14.9) | 981 (14.5) | 5133 (1.5) | 17,073 (3.0) | 757 (6.3) | ||
| ≥3 times/week | 20,471 (14.4) | 158,434 (24.8) | 2955 (46.8) | 4540 (1.4) | 16,018 (2.8) | 879 (7.3) | ||
| Systolic BP, mmHg | 127.5 ±15.1 | 128.1 ±15.1 | 130.9 ±16.3 | <0.0001 * | 127.5 ±16.1 | 126.3 ±16.1 | 126.3 ±16.3 | <0.0001 ‡ |
| Diastolic BP, mmHg | 78.3 ±10.0 | 79.0 ±9.9 | 80.6 ±10.4 | <0.0001 * | 77.5 ±10.0 | 77.2 ±10.0 | 77.8 ±10.3 | <0.0001 † |
| Hypertension, n (%) | 94,843 (66.4) | 398,574 (62.2) | 3890 (61.3) | <0.0001 ‡ | 215,857 (64.5) | 325,151 (56.6) | 6192 (51.1) | <0.0001 * |
| DM, n (%) | 47,168 (33.0) | 161,636 (25.2) | 1421 (22.4) | <0.0001 * | 84,362 (25.2) | 102,899 (17.9) | 1801 (14.9) | <0.0001 * |
| Blood test at baseline | ||||||||
| FBS, mg/dL | 101 [91–118] | 99 [90–113] | 100 [90–113] | <0.0001 ‡ | 97 [89–110] | 96 [88–106] | 95 [87–105] | <0.0001 * |
| sCr, mg/dL | 1.0 [0.9–1.2] | 1.0 [0.9–1.1] | 1.0 [0.9–1.1] | <0.0001 * | 0.8 [0.7–0.9] | 0.8 [0.7–0.9] | 0.8 [0.7–0.9] | <0.0001 ‡ |
| TC, mg/dL | 174 [149–199] | 189 [165–215] | 212 [188–239] | <0.0001 * | 191 [166–218] | 203 [179–230] | 224 [199–252] | <0.0001 * |
| LDL-C, mg/dL | 102 [79–125] | 108 [85–131] | 91 [67–117] | <0.0001 * | 116 [93–141] | 118 [96–143] | 106 [82–133] | <0.0001 * |
| HDL-C, mg/dL | 35 [32–38] | 52 [45–60] | 97 [93–105] | <0.0001 * | 43 [39–46] | 60 [54–67] | 97 [93–103] | <0.0001 * |
| Triglycerides, mg/dL | 164 [115–237] | 121 [86–175] | 89 [64–129] | <0.0001 * | 145 [104–202] | 106 [77–145] | 83 [61–116] | <0.0001 * |
| Non-HDL-C, mg/dL | 139 [115–165] | 135 [111–161] | 111 [86–138] | <0.0001 * | 149 [124–175] | 142 [118–168] | 125 [100–153] | <0.0001 * |
| All Subjects | Total (N = 1,711,548) | Low (N = 477,346) | High (N = 1,215,741) | Extremely High (N = 18,461) | p Value |
|---|---|---|---|---|---|
| Total, n (%) | 218,252 (12.8) | 68,615 (14.4) | 147,243 (12.1) | 2394 (13.0) | <0.0001 * |
| By age, n (% of each age subgroup) | |||||
| 40–49 years | 4379 (1.9) | 1037 (2.1) | 3248 (1.9) | 94 (3.4) | <0.0001 * |
| 50–59 years | 19,237 (3.7) | 4835 (3.7) | 14,108 (3.7) | 294 (4.7) | <0.0001 † |
| 60–69 years | 53,495 (10.0) | 15,091 (9.8) | 37,774 (10.1) | 630 (11.8) | <0.0001 * |
| 70–79 years | 99,699 (27.6) | 32,118 (27.1) | 66,577 (27.8) | 1004 (29.6) | <0.0001 * |
| ≥80 years | 41,442 (61.5) | 15,534 (62.1) | 25,536 (61.1) | 372 (57.9) | 0.0068 † |
| Males | Total (N = 790,040) | Low (N = 142,893) | High (N = 640,800) | Extremely High (N = 6347) | p value |
| Male, n (%) | 127,821 (16.2) | 27,782 (19.4) | 98666 (15.4) | 1373 (21.6) | <0.0001 * |
| By age, n (% of each age subgroup) | |||||
| 40–49 years, n (%) | 3188 (2.6) | 631 (3.1) | 2498 (2.5) | 59 (7.3) | <0.0001 * |
| 50–59 years, n (%) | 13,695 (5.5) | 2824 (6.6) | 10,671 (5.3) | 200 (10.4) | <0.0001 * |
| 60–69 years, n (%) | 35,587 (14.7) | 7532 (17.3) | 27,651 (14.0) | 404 (19.1) | <0.0001 * |
| 70–79 years, n (%) | 56,710 (37.3) | 12,658 (41.9) | 43,492 (36.1) | 560 (43.3) | <0.0001 * |
| ≥80 years, n (%) | 18,641 (70.7) | 4137 (74.6) | 14,354 (69.7) | 150 (69.4) | <0.0001 ‡ |
| Females | Total (N = 921,508) | Low (N = 334,453) | High (N = 574,941) | Extremely High (N = 12,114) | p value |
| Female, n (%) | 90,431 (9.8) | 40,833 (12.2) | 48577 (8.4) | 1021 (8.4) | <0.0001 ‡ |
| By age, n (% of each age subgroup) | |||||
| 40–49 years, n (%) | 1191 (1.1) | 406 (1.4) | 750 (1.0) | 35 (1.8) | <0.0001 * |
| 50–59 years, n (%) | 5542 (2.0) | 2011 (2.3) | 3437 (1.9) | 94 (2.1) | <0.0001 § |
| 60–69 years, n (%) | 17,908 (6.2) | 7559 (6.8) | 10,123 (5.7) | 226 (7.0) | <0.0001 || |
| 70–79 years, n (%) | 42,989 (20.5) | 19,460 (22.0) | 23,085 (19.4) | 444 (21.2) | <0.0001 || |
| ≥80 years, n (%) | 22,801 (55.5) | 11,397 (58.5) | 11,182 (52.8) | 222 (52.0) | <0.0001 ‡ |
| Sex | Reference HDL-C | Group of HDL-C | Unadjusted HR (95% CI) | p Value | Adjusted HR * (95% CI) | p Value * |
|---|---|---|---|---|---|---|
| Male | 40–90 mg/dL | Low | 1.296 (1.279–1.314) | <0.0001 | 1.183 (1.166–1.199) | <0.0001 |
| Extremely high | 1.464 (1.388–1.545) | <0.0001 | 1.359 (1.288–1.434) | <0.0001 | ||
| Female | 50–90 mg/dL | Low | 1.472 (1.453–1.492) | <0.0001 | 1.153 (1.138–1.169) | <0.0001 |
| Extremely high | 0.994 (0.934–1.053) | 0.853 | 1.095 (1.029–1.167) | 0.0041 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.S.; Jeong, H.J.; Kim, H.; Lee, S.; Hur, M. Sex-Specific Relationships between HDL-Cholesterol Levels and 10-Year Mortality in Individuals with Atherosclerotic Cardiovascular Disease: A Nationwide Cohort Study of South Koreans. Metabolites 2023, 13, 1175. https://doi.org/10.3390/metabo13121175
Yang HS, Jeong HJ, Kim H, Lee S, Hur M. Sex-Specific Relationships between HDL-Cholesterol Levels and 10-Year Mortality in Individuals with Atherosclerotic Cardiovascular Disease: A Nationwide Cohort Study of South Koreans. Metabolites. 2023; 13(12):1175. https://doi.org/10.3390/metabo13121175
Chicago/Turabian StyleYang, Hyun Suk, Ho Jin Jeong, Hyeongsu Kim, Seungho Lee, and Mina Hur. 2023. "Sex-Specific Relationships between HDL-Cholesterol Levels and 10-Year Mortality in Individuals with Atherosclerotic Cardiovascular Disease: A Nationwide Cohort Study of South Koreans" Metabolites 13, no. 12: 1175. https://doi.org/10.3390/metabo13121175
APA StyleYang, H. S., Jeong, H. J., Kim, H., Lee, S., & Hur, M. (2023). Sex-Specific Relationships between HDL-Cholesterol Levels and 10-Year Mortality in Individuals with Atherosclerotic Cardiovascular Disease: A Nationwide Cohort Study of South Koreans. Metabolites, 13(12), 1175. https://doi.org/10.3390/metabo13121175