In Vitro Anti-Oxidant, In Vivo Anti-Hyperglycemic, and Untargeted Metabolomics-Aided-In Silico Screening of Macroalgae Lipophilic Extracts for Anti-Diabetes Mellitus and Anti-COVID-19 Potential Metabolites
Abstract
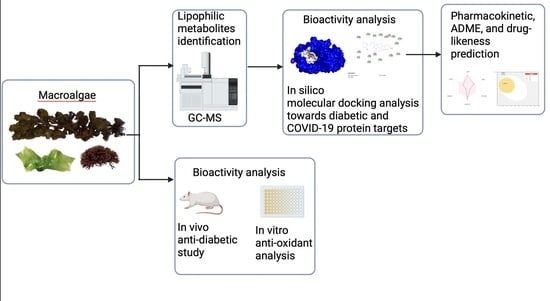
:1. Introduction
2. Materials and Methods
2.1. Sample Collection, Preparation, and Lipophilic Compounds Extraction
2.2. In Vivo Anti-Diabetes Analysis in an Alloxan-Induced Diabetic Mouse Model
2.3. In Vitro Anti-Oxidant Analysis
2.3.1. DPPH Assay
2.3.2. ABTS Assay
2.4. Untargeted Gas Chromatography-Mass Spectrometry (GC-MS) Metabolomics Analysis
2.5. In Silico Analysis of Anti-Diabetic Activity and Anti-COVID-19 Activity
3. Results
3.1. In Vivo Anti-Hyperglycemic Activity of Sargassum Cristaefolium, Tricleocarpa cylindrica, and Ulva lactuca Lipophilic Extract
3.2. In Vitro Anti-Oxidant Activity of Sargassum cristaefolium, Tricleocarpa cylindrica, and Ulva lactuca Lipophilic Extract
3.3. Untargeted GC-MS Metabolomics Analysis of Macroalgae Lipophilic Extract
3.4. In Silico Molecular Docking Analysis
3.5. Analysis of Physiochemical Properties and ADME Studies of the Compounds
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Birman, D. Investigation of the Effects of COVID-19 on Different Organs of the Body. Eurasian J. Chem. Med. Pet. Res. 2022, 2, 24–36. [Google Scholar]
- Yan, Y.; Yang, Y.; Wang, F.; Ren, H.; Zhang, S.; Shi, X.; Xiaoli, C.; Xuefeng, Y.; Dong, K. Clinical characteristics and outcomes of patients with severe COVID-19 with diabetes. BMJ Open Diabetes Res. Care 2020, 8, e001343. [Google Scholar] [CrossRef] [PubMed]
- Shevchuk, O.; Park, A.; Palii, S.; Ivankiv, Y.; Kozak, K.; Korda, M.; Vari, S.G. Blood ACE2 protein level correlates with COVID-19 severity. Int. J. Mol. Sci. 2023, 24, 13957. [Google Scholar] [CrossRef] [PubMed]
- Erener, S. Diabetes, infection risk and COVID-19. Mol. Metab. 2020, 39, 101044. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Karakiulakis, G.; Roth, M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir. Med. 2020, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Muniyappa, R.; Gubbi, S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E736–E741. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Lau, A.; So, H.C. Exploring diseases/traits and blood proteins causally related to expression of ACE2, the putative receptor of SARS-CoV-2: A Mendelian randomization analysis highlights tentative relevance of diabetes-related traits. Diabetes Care 2020, 43, 1416–1426. [Google Scholar] [CrossRef]
- Soldo, J.; Heni, M.; Königsrainer, A.; Häring, H.U.; Birkenfeld, A.L.; Peter, A. Increased hepatic ACE2 expression in NAFL and diabetes—A risk for COVID-19 patients? Diabetes Care 2020, 43, e134–e136. [Google Scholar] [CrossRef]
- Brufsky, A. Hyperglycemia, hydroxychloroquine, and the COVID-19 pandemic. J. Med. Virol. 2020, 92, 770–775. [Google Scholar] [CrossRef]
- Mehdipour, A.R.; Hummer, G. Dual nature of human ACE2 glycosylation in binding to SARS-CoV-2 spike. Proc. Natl. Acad. Sci. USA 2021, 118, e2100425118. [Google Scholar] [CrossRef]
- Couselo-Seijas, M.; Almengló, C.; Agra-Bermejo, R.M.; Luis Fernandez, A.; Alvarez, E.; R González-Juanatey, J.; Eiras, S. Higher ACE2 Expression Levels in Epicardial Cells Than Subcutaneous Stromal Cells from Patients with Cardiovascular Disease: Diabetes and Obesity as Possible Enhancer. Eur. J. Clin. Investig. 2021, 51, e13463. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Li, M.; Dong, Y.; Zhou, H.; Zhang, Z.; Tian, C.; Qin, R.; Wang, H.; Shen, Y.; Du, K.; et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes/Metab. Res. Rev. 2020, 36, 3319. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.J.; Goddard-Borger, E.D. α-glucosidase inhibitors as host-directed antiviral agents with potential for the treatment of COVID-19. Biochem. Soc. Trans. 2020, 48, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.N.; Ho, D.S.; Nguyen, H.S.; Ho, D.K.N.; Li, H.Y.; Lin, C.Y.; Chiu, H.Y.; Chen, Y.C. Preadmission use of antidiabetic medications and mortality among patients with COVID-19 having type 2 diabetes: A meta-analysis. Metabolism 2022, 131, 155196. [Google Scholar] [CrossRef] [PubMed]
- Chiasson, J.L.; Josse, R.G.; Gomis, R.; Hanefeld, M.; Karasik, A.; Laakso, M. Acarbose for prevention of type 2 diabetes mellitus: The STOP-NIDDM randomised trial. Lancet 2002, 359, 2072–2077. [Google Scholar] [CrossRef] [PubMed]
- Dabhi, A.S.; Bhatt, N.R.; Shah, M.J. Voglibose: An alpha glucosidase inhibitor. J. Clin. Diagn. Res. 2013, 7, 3023. [Google Scholar] [PubMed]
- Popovic-Djordjevic, J.B.; Jevtic, I.I.; Stanojkovic, T.P. Antidiabetics: Structural diversity of molecules with a common aim. Curr. Med. Chem. 2018, 25, 2140–2165. [Google Scholar] [CrossRef]
- Prasedya, E.S.; Padmi, H.; Ilhami, B.T.K.; Martyasari, N.W.R.; Sunarwidhi, A.L.; Widyastuti, S.; Khairinisa, M.A.; Cokrowati, N.; Simangunsong, E.E.; Frediansyah, A. Brown macroalgae Sargassum cristaefolium extract inhibits melanin production and cellular oxygen stress in B16F10 melanoma cells. Molecules 2022, 27, 8585. [Google Scholar] [CrossRef]
- Prasedya, E.S.; Martyasari, N.W.R.; Ilhami, B.T.K.; Padmi, H.; Widyastuti, S.; Sunarwidhi, A.L.; Sunarpi, H. Antiaging effect of brown macroalgae Sargassum cristaefolium gel formulation by inhibition of collagen degradation. IOP Conf. Ser. Earth Environ. Sci. 2021, 712, 012030. [Google Scholar] [CrossRef]
- Sunarwidhi, A.L.; Hernawan, A.; Utami, N.W.P.; Maulana, F.A.; Ichfa, M.S.M.; Handayani, E.; Rosyantari, A.; Ambana, Y.; Prasedya, E.S.; Widyastuti, S. The lipid extract of Sargassum polycystum found in Lombok coast Indonesia acts against free radicals and diabetes mellitus. AIP Conf. Proc. 2023, 2956, 040010. [Google Scholar]
- Murray, M.; Dordevic, A.L.; Ryan, L.; Bonham, M.P. An emerging trend in functional foods for the prevention of cardiovascular disease and diabetes: Marine algal polyphenols. Crit. Rev. Food Sci. Nutr. 2018, 58, 1342–1358. [Google Scholar] [CrossRef] [PubMed]
- Terme, N.; Boulho, R.; Kucma, J.P.; Bourgougnon, N.; Bedoux, G. Radical scavenging activity of lipids from seaweeds isolated by solid-liquid extraction and supercritical fluids. OCL 2018, 25, D505. [Google Scholar] [CrossRef]
- Kim, S.K.; Van Ta, Q. Potential beneficial effects of marine algal sterols on human health. Adv. Food Nutr. Res. 2011, 64, 191–198. [Google Scholar] [PubMed]
- Rodeiro, I.; Olguín, S.; Santes, R.; Herrera, J.A.; Pérez, C.L.; Mangas, R.; Hernández, Y.; Fernández, G.; Hernández, I.; Hernández-Ojeda, S.; et al. Gas chromatography-mass spectrometry analysis of Ulva fasciata (Green seaweed) extract and evaluation of its cytoprotective and antigenotoxic effects. Evid.-Based Complement. Altern. Med. 2015, 2015, 520598. [Google Scholar] [CrossRef] [PubMed]
- Sunarwidhi, A.L.; Hernawan, A.; Frediansyah, A.; Widyastuti, S.; Martyasari, N.W.R.; Abidin, A.S.; Padmi, H.; Handayani, E.; Utami, N.W.P.; Maulana, F.A.; et al. Multivariate Analysis Revealed Ultrasonic-Assisted Extraction Improves Anti-Melanoma Activity of Non-Flavonoid Compounds in Indonesian Brown Algae Ethanol Extract. Molecules 2022, 27, 7509. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.Z.; Li, T.T.; Zhong, R.T.; Chen, H.B.; Xia, X.; Gao, L.Y.; Gao, X.X.; Liu, B.; Zhang, H.Y.; Zhao, C. Anti-diabetic activity of PUFAs-rich extracts of Chlorella pyrenoidosa and Spirulina platensis in rats. Food Chem. Toxicol. 2019, 128, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Abd El Hafez, M.S.; Aziz Okbah, M.A.E.; Ibrahim, H.A.; Hussein, A.A.E.R.; El Moneim, N.A.A.; Ata, A. First report of steroid derivatives isolated from starfish Acanthaster planci with anti-bacterial, anti-cancer and anti-diabetic activities. Nat. Prod. Res. 2022, 36, 5545–5552. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Um, B.H.; Kim, S.M. Two unsaturated fatty acids with potent α-glucosidase inhibitory activity purified from the body wall of sea cucumber (Stichopus japonicus). J. Food Sci. 2011, 76, H208–H214. [Google Scholar] [CrossRef]
- Aletor, V.A.; Eder, K.; Becker, K.; Paulicks, B.R.; Roth, F.X.; Roth-Maier, D.A. The effects of conjugated linoleic acids or an alpha-glucosidase inhibitor on tissue lipid concentrations and fatty acid composition of broiler chicks fed a low-protein diet. Poult. Sci. 2003, 82, 796–804. [Google Scholar] [CrossRef]
- Artanti, N.; Tachibana, S.; Kardono, L.B.; Sukiman, H. Isolation of alpha-glucosidase inhibitors produced by an endophytic fungus, Colletotrichum sp. TSC13 from Taxus sumatrana. Pak. J. Biol. Sci. PJBS 2012, 15, 673–679. [Google Scholar] [CrossRef]
- Liu, B.; Kongstad, K.T.; Wiese, S.; Jäger, A.K.; Staerk, D. Edible seaweed as future functional food: Identification of α-glucosidase inhibitors by combined use of high-resolution α-glucosidase inhibition profiling and HPLC–HRMS–SPE–NMR. Food Chem. 2016, 203, 16–22. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Ramenskaya, G.; Kustrin, E.; Morton, D.W. Characterisation of α-amylase inhibitors in marigold plants via bioassay-guided high-performance thin-layer chromatography and attenuated total reflectance–Fourier transform infrared spectroscopy. J. Chromatogr. B 2021, 1173, 122676. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, M.; Loizzo, M.R.; Nicoletti, M.; Menichini, F.; Conforti, F. Inhibition of key enzymes linked to obesity by preparations from Mediterranean dietary plants: Effects on α-amylase and pancreatic lipase activities. Plant Foods Hum. Nutr. 2013, 68, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Baral, P.K.; Amin, M.T.; Rashid, M.M.O.; Hossain, M.S. Assessment of polyunsaturated fatty acids on COVID-19-associated risk reduction. Rev. Bras. Farmacogn. 2022, 32, 50–64. [Google Scholar] [CrossRef] [PubMed]
- Payghami, N.; Jamili, S.; Rustaiyan, A.; Saeidnia, S.; Nikan, M.; Gohari, A.R. Alpha-amylase inhibitory activity and sterol composition of the marine algae, Sargassum glaucescens. Pharmacogn. Res. 2015, 7, 314. [Google Scholar]
- Sheng, Z.; Dai, H.; Pan, S.; Ai, B.; Zheng, L.; Zheng, X.; Prinyawiwatkul, W.; Xu, Z. Phytosterols in banana (Musa spp.) flower inhibit α-glucosidase and α-amylase hydrolysations and glycation reaction. Int. J. Food Sci. Technol. 2017, 52, 171–179. [Google Scholar] [CrossRef]
- Ye, X.; Song, C.; Yuan, P.; Mao, R. α-Glucosidase and α-amylase inhibitory activity of common constituents from traditional Chinese medicine used for diabetes mellitus. Chin. J. Nat. Med. 2010, 8, 349–352. [Google Scholar] [CrossRef]
- Hisham Shady, N.; Youssif, K.A.; Sayed, A.M.; Belbahri, L.; Oszako, T.; Hassan, H.M.; Abdelmohsen, U.R. Sterols and triterpenes: Antiviral potential supported by in-silico analysis. Plants 2020, 10, 41. [Google Scholar] [CrossRef]
- Ramu, R.; Shirahatti, P.S.; Nayakavadi, S.; Vadivelan, R.; Zameer, F.; Dhananjaya, B.L.; Prasad, N. The effect of a plant extract enriched in stigmasterol and β-sitosterol on glycaemic status and glucose metabolism in alloxan-induced diabetic rats. Food Funct. 2016, 7, 3999–4011. [Google Scholar] [CrossRef]
- Pacheco, B.S.; Dos Santos, M.A.Z.; Schultze, E.; Martins, R.M.; Lund, R.G.; Seixas, F.K.; Colepicolo, P.; Collares, T.; Paula, F.R.; De Pereira, C.M.P. Cytotoxic activity of fatty acids from Antarctic macroalgae on the growth of human breast cancer cells. Front. Bioeng. Biotechnol. 2018, 6, 185. [Google Scholar] [CrossRef]
- Zhao, C.; Yang, C.; Liu, B.; Lin, L.; Sarker, S.D.; Nahar, L.; Yu, H.; Cao, H.; Xiao, J. Bioactive compounds from marine macroalgae and their hypoglycemic benefits. Trends Food Sci. Technol. 2018, 72, 1–12. [Google Scholar] [CrossRef]
- Tang, H.F.; Yi, Y.H.; Yao, X.S.; Xu, Q.Z.; Zhang, S.Y.; Lin, H.W. Bioactive steroids from the brown alga Sargassum carpophyllum. J. Asian Nat. Prod. Res. 2002, 4, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Ktari, L.; Blond, A.; Guyot, M. 16β-Hydroxy-5α-cholestane-3, 6-dione, a novel cytotoxic oxysterol from the red alga Jania rubens. Bioorg. Med. Chem. Lett. 2000, 10, 2563–2565. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.S.; Engel, S.; Smith, B.A.; Fairchild, C.R.; Aalbersberg, W.; Hay, M.E.; Kubanek, J. Structure and biological evaluation of novel cytotoxic sterol glycosides from the marine red alga Peyssonnelia sp. Bioorg. Med. Chem. 2010, 18, 8264–8269. [Google Scholar] [CrossRef] [PubMed]
- Plaza, M.; Cifuentes, A.; Ibáñez, E. In the search of new functional food ingredients from algae. Trends Food Sci. Technol. 2008, 19, 31–39. [Google Scholar] [CrossRef]
- Lee, S.; Lee, Y.S.; Jung, S.H.; Kang, S.S.; Shin, K.H. Anti-oxidant activities of fucosterol from the marine algae Pelvetia siliquosa. Arch. Pharmacal Res. 2003, 26, 719–722. [Google Scholar] [CrossRef]
- Jung, H.A.; Islam, M.N.; Lee, C.M.; Oh, S.H.; Lee, S.; Jung, J.H.; Choi, J.S. Kinetics and molecular docking studies of an anti-diabetic complication inhibitor fucosterol from edible brown algae Eisenia bicyclis and Ecklonia stolonifera. Chem. -Biol. Interact. 2013, 206, 55–62. [Google Scholar] [CrossRef]
- Hagiwara, H.; Wakita, K.I.; Inada, Y.; Hirose, S. Fucosterol decreases angiotensin converting enzyme levels with reduction of glucocorticoid receptors in endothelial cells. Biochem. Biophys. Res. Commun. 1986, 139, 348–352. [Google Scholar] [CrossRef]
- Luo, S.K.; Hu, W.H.; Lu, Z.J.; Li, C.; Fan, Y.M.; Chen, Q.J.; Chen, Z.S.; Ye, J.F.; Chen, S.Y.; Tong, J.L.; et al. Diabetes patients with comorbidities had unvaforable outcomes following COVID-19: A retrospective study. World J. Diabetes 2020, 12, 1789. [Google Scholar] [CrossRef]
- Algaebase. Listing the World’s Algae. 2021. Available online: https://www.algaebase.org/ (accessed on 1 April 2023).
- Prasedya, E.S.; Martyasari, N.W.R.; Abidin, A.S.; Pebriani, S.A.; Ilhami, B.T.K.; Frediansyah, A.; Sunarwidhi, A.L.; Widyastuti, S.; Sunarpi, H. Macroalgae Sargassum cristaefolium extract inhibits proinflammatory cytokine expression in BALB/C Mice. Scientifica 2020, 2020, 9769454. [Google Scholar] [CrossRef]
- Liu, S.L.; Lin, S.M.; Chen, P.C. Phylogeny, species diversity and biogeographic patterns of the genus Tricleocarpa (Galaxauraceae, Rhodophyta) from the Indo-Pacific region, including T. confertus sp. nov. from Taiwan. Eur. J. Phycol. 2015, 50, 439–456. [Google Scholar] [CrossRef]
- Prasedya, E.S.; Martyasari, N.W.R.; Apriani, R.; Mayshara, S.; Fanani, R.A.; Sunarpi, H. Antioxidant activity of Ulva lactuca L. from different coastal locations of Lombok Island, Indonesia. AIP Conf. Proc. 2019, 2199, 020003. [Google Scholar]
- Prasedya, E.S.; Frediansyah, A.; Martyasari, N.W.R.; Ilhami, B.K.; Abidin, A.S.; Padmi, H.; Fahrurrozi Juanssilfero, A.B.; Widyastuti, S.; Sunarwidhi, A.L. Effect of particle size on phytochemical composition and antioxidant properties of Sargassum cristaefolium ethanol extract. Sci. Rep. 2021, 11, 17876. [Google Scholar] [CrossRef] [PubMed]
- Aba, P.E.; Igwebuike, D.C.; Onah, J.A. Effects of various concentrations of quail egg solution on glycemia and antioxidant parameters of alloxan-induced diabetic rats. J. Adv. Med. Pharm. Sci. 2015, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nunes, P.R.; Bueno Pereira, T.O.; Bertozzi Matheus, M.; Grandini, N.A.; Siqueira, J.S.; Correa, C.R.; Abbade, J.F.; Sandrim, V.C. Glibenclamide Increases Nitric Oxide Levels and Decreases Oxidative Stress in an In Vitro Model of Preeclampsia. Antioxidants 2022, 11, 1620. [Google Scholar] [CrossRef] [PubMed]
- Sunarwidhi, A.L.; Rosyantari, A.; Prasedya, E.S.; Ardiana, N.; Ilhami, B.T.K.; Abidin, A.S.; Ambana, Y.; Kirana, I.A.P.; Wirasisya, D.G.; Anugrah, W.; et al. The correlation between total protein content and antioxidant activity of collagen isolated from a marine sponge Stylissa flabelliformis collected from North Lombok Indonesia coast. IOP Conf. Ser. Earth Environ. Sci. 2021, 913, 012103. [Google Scholar] [CrossRef]
- Nenadis, N.; Wang, L.-F.; Tsimidou, M.; Zhang, H.-Y. Estimation of scavenging activity of phenolic compounds using the ABTS·+ assay. J. Agric. Food Chem. 2004, 52, 4669–4674. [Google Scholar] [CrossRef] [PubMed]
- Biovia, D.S.; Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Richmond, T.J. Dassault Systèmes BIOVIA, Discovery Studio Visualizer, 17.2; Dessault Systemes: San Diego, CA, USA, 2016; Volume 10. [Google Scholar]
- Informatics, P. ChemDraw User Guide; Copyright 1998–2017 PerkinElmer Informatics Inc., All rights reserved; PerkinElmer Informatics Inc.: Waltham, MA, USA, 2017; pp. 1–368. [Google Scholar]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Schrödinger, L.; DeLano, W. PyMOL. 2020. Available online: http://www.pymol.org/pymol (accessed on 14 June 2023).
- Kosanić, M.; Ranković, B.; Stanojković, T. Biological potential of marine macroalgae of the genus Cystoseira. Acta Biol. Hung. 2015, 66, 374–384. [Google Scholar] [CrossRef]
- Pirian, K.; Moein, S.; Sohrabipour, J.; Rabiei, R.; Blomster, J. Antidiabetic and antioxidant activities of brown and red macroalgae from the Persian Gulf. J. Appl. Phycol. 2017, 29, 3151–3159. [Google Scholar] [CrossRef]
- Mansour, D.S.; Mousa, A.M. Ameliorative potential role of Rosmarinus officinalis extract on toxicity induced by etoposide in male albino rats. Braz. J. Biol. 2022, 84, e258234. [Google Scholar]
- Abdelbasset, W.K.; Jasim, S.A.; Rudiansyah, M.; Huldani, H.; Margiana, R.; Jalil, A.T.; Mohammad, H.J.; Ridha, H.; Yasin, G. Treatment of pilocarpine-induced epileptic seizures in adult male mice. Braz. J. Biol. 2022, 84, e260091. [Google Scholar] [CrossRef] [PubMed]
- Neamtu, A.; Mocci, F.; Laaksonen, A.; da Silva, F.L.B. Towards an optimal monoclonal antibody with higher binding affinity to the receptor-binding domain of SARS-CoV-2 spike proteins from different variants. Colloids Surf. B Biointerfaces 2023, 221, 112986. [Google Scholar] [CrossRef]
- Campbell, L.K.; White, J.R.; Campbell, R.K. Acarbose: Its role in the treatment of diabetes mellitus. Ann. Pharmacother. 1996, 30, 1255–1262. [Google Scholar] [CrossRef]
- Jia, H.; Neptune, E.; Cui, H. Targeting ACE2 for COVID-19 therapy: Opportunities and challenges. Am. J. Respir. Cell Mol. Biol. 2021, 64, 416–425. [Google Scholar] [CrossRef]
- Bestle, D.; Heindl, M.R.; Limburg, H.; Pilgram, O.; Moulton, H.; Stein, D.A.; Hardes, K.; Eickmann, M.; Dolnik, O.; Rohde, C.; et al. TMPRSS2 and furin are both essential for proteolytic activation of SARS-CoV-2 in human airway cells. Life Sci. Alliance 2020, 3, e202000786. [Google Scholar] [CrossRef]
- Lukassen, S.; Chua, R.L.; Trefzer, T.; Kahn, N.C.; Schneider, M.A.; Muley, T.; Winter, H.; Meister, M.; Veith, C.; Boots, A.W.; et al. SARS-CoV-2 receptor ACE 2 and TMPRSS 2 are primarily expressed in bronchial transient secretory cells. EMBO J. 2020, 39, e105114. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020, 181, 271–280. [Google Scholar] [CrossRef]
- Shang, J.; Wan, Y.; Luo, C.; Ye, G.; Geng, Q.; Auerbach, A.; Li, F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 11727–11734. [Google Scholar] [CrossRef]
- Al-Gara, N.I.; Abu-Serag, N.A.; Alee Shaheed, K.A.; Bahadly, Z.K.A. Analysis of bioactive phytochemical compound of (Cyperus alternifolius L.) By using gas chromatography–mass spectrometry. IOP Conf. Ser. Mater. Sci. Eng. 2019, 571, 0123047. [Google Scholar] [CrossRef]
- Salem, S.H.; El-Maraghy, S.S.; Abdel-Mallek, A.Y.; Abdel-Rahman, M.A.; Hassanein, E.H.; Al-Bedak, O.A.; Sayed, A.M. GC–MS analysis, cytotoxicity, and molecular docking studies of bioactive alkaloids extracted from tomato leaves inoculated with endophytic fungus Beauveria sp. AUMC 15401. J. Food Process. Preserv. 2022, 46, e17039. [Google Scholar] [CrossRef]
- Sympli, H.D. Estimation of drug-likeness properties of GC–MS separated bioactive compounds in rare medicinal Pleione maculata using molecular docking technique and SwissADME in silico tools. Netw. Model. Anal. Health Inform. Bioinform. 2021, 10, 14. [Google Scholar] [CrossRef]
- Gill, H.S.; Londowski, J.M.; Corradino, R.A.; Zinsmeister, A.R.; Kumar, R. The synthesis and biological activity of 25-hydroxy-26, 27-dimethylvitamin D3 and 1, 25-dihydroxy-26, 27-dimethylvitamin D3: Highly potent novel analogs of vitamin D3. J. Steroid Biochem. 1988, 31, 147–160. [Google Scholar] [CrossRef]
- Babar, A.G.; Pande, A.; Kulkarni, B.G. Anti-fungal activity and investigation of bioactive compounds of marine intertidal bivalve Gafrarium divaricatum from West coast of India. Int. J. Pure Appl. Biosci. 2016, 4, 211–217. [Google Scholar] [CrossRef]
- AlAmery, S.F. Phytochemical profile and antifungal activity of stems and leaves methanol extract from the Juncus maritimus Linn. Juncaceae family against some dermatophytes fungi. AIP Conf. Proc. 2020, 2290, 020034. [Google Scholar]
- Rautela, I.; Dheer, P.; Thapliyal, P.; Joshi, T.; Sharma, N.; Sharma, M.D. GC-MS analysis of plant leaf extract of Datura stramonium in different solvent system. Eur. J. Biomed. Pharm. Sci. 2018, 5, 236–245. [Google Scholar]
- Lee, Y.S.; Shin, K.H.; Kim, B.K.; Lee, S. Anti-diabetic activities of fucosterol from Pelvetia siliquosa. Arch. Pharmacal Res. 2004, 27, 1120–1122. [Google Scholar] [CrossRef]
- Ghosh, T.; Maity, T.K.; Singh, J. Evaluation of antitumor activity of stigmasterol, a constituent isolated from Bacopa monnieri Linn aerial parts against Ehrlich Ascites Carcinoma in mice. Orient. Pharm. Exp. Med. 2011, 11, 41–49. [Google Scholar] [CrossRef]
- Sureshkumar, P.; Senthilraja, P.; Kalavathy, S. In-silico docking analysis of Calotropisgigantea (L). R Br derived compound against anti-cervical cancer activity. World Res. J. Comput. Aided Drug Des. 2012, 1, 9–12. [Google Scholar]
- Loori, M.; Sourinejad, I.; Nazemi, M. Identification and investigation of antibacterial effects of steroidal fraction from the marine sponge Axinella sinoxea Alvarez & Hooper, 2009 in Larak island, the Persian Gulf. Fish. Sci. Technol. 2021, 10, 164–172. [Google Scholar]
- Doak, B.C.; Over, B.; Giordanetto, F.; Kihlberg, J. Oral druggable space beyond the rule of 5: Insights from drugs and clinical candidates. Chem. Biol. 2014, 21, 1115–1142. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Piccaro, G.; Poce, G.; Biava, M.; Giannoni, F.; Fattorini, L. Activity of lipophilic and hydrophilic drugs against dormant and replicating Mycobacterium tuberculosis. J. Antibiot. 2015, 68, 711–714. [Google Scholar] [CrossRef] [PubMed]
- Makarov, V.; Lechartier, B.; Zhang, M.; Neres, J.; van der Sar, A.M.; Raadsen, S.A.; Hartkoorn, R.C.; Ryabova, O.B.; Vocat, A.; Decosterd, L.A.; et al. Towards a new combination therapy for tuberculosis with next generation benzothiazinone. EMBO Mol. Med. 2014, 6, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Azzi, A. Oxidative stress: What is it? Can it be measured? Where is it located? Can it be good or bad? Can it be prevented? Can it be cured? Antioxidants 2022, 11, 1431. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, S.; Khan, A. Antioxidants and diabetes. Indian J. Endocrinol. Metab. 2012, 16 (Suppl. S2), S267. [Google Scholar] [CrossRef]
- Ceriello, A.; Testa, R. Antioxidant anti-inflammatory treatment in type 2 diabetes. Diabetes Care 2009, 32 (Suppl. S2), S232. [Google Scholar] [CrossRef]
- Yim, S.; Malhotra, A.; Veves, A. Antioxidants and CVD in diabetes: Where do we stand now? Curr. Diabetes Rep. 2007, 7, 8–13. [Google Scholar] [CrossRef]
- Ceriello, A.; Testa, R.; Genovese, S. Clinical implications of oxidative stress and potential role of natural antioxidants in diabetic vascular complications. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 285–292. [Google Scholar] [CrossRef]
- Sankhla, M.; Sharma, T.K.; Mathur, K.; Rathor, J.S.; Butolia, V.; Gadhok, A.K.; Vardey, S.K.; Sinha, M.; Kaushik, G.G. Relationship of oxidative stress with obesity and its role in obesity induced metabolic syndrome. Clin. Lab. 2012, 58, 385–392. [Google Scholar] [PubMed]
- Kositsawat, J.; Freeman, V.L. Vitamin C and A1c relationship in the national health and nutrition examination Survey (NHANES) 2003–2006. J. Am. Coll. Nutr. 2011, 30, 477–483. [Google Scholar] [CrossRef] [PubMed]










| Sample | IC50 (μg/mL) ± SEM | IC50 (μg/mL) ± SEM |
|---|---|---|
| DPPH | ABTS | |
| Sargassum cristaefolium | 206.7 ± 0.11 a | 200.47 ± 0.09 a |
| Tricleocarpa cylindrica | 252 ± 0.10 b | 353.22 ± 0.07 a,b |
| Ulva lactuca | 308.6 ± 0.13 b | 386.42 ± 0.05 a,b |
| Ascorbic acid | 3.67 ± 0.02 | 5.58 ± 0.02 |
| Sargassum cristaefolium | ||||||
|---|---|---|---|---|---|---|
| No. | Ret. Time (min) | Compound Name | Compound Classification | Chemical Formula | Mol. Weight | Peak Area (%) |
| 1 | 10.17 | Dodecanoic acid, 3-hydroxy-(3-hydroxy lauric acid) | Fatty acid | C12H24O3 | 216 | 0.28 |
| 2 | 10.98 | 13-heptadecyn-1-ol | Fatty alcohol | C17H32O | 252 | 0.39 |
| 3 | 11.4 | Heptadecane | Alkane hydrocarbon | C17H36 | 240 | 5.6 |
| 4 | 11.53 | 7-methyl-Z-tetradecen-1-ol acetate | Ester | C17H32O2 | 268 | 0.21 |
| 5 | 11.69 | Cyclopropanebutanoic acid, 2-[[2-[[2-[(2-pentyl cyclopropyl) methyl] methyl ester | Fatty acids methyl ester | C25H42O2 | 374 | 0.24 |
| 6 | 13.03 | Neophytadiene | Terpenoid | C20H38 | 278 | 8.42 |
| 7 | 13.36 | 3,7,11,15-tetramethyl-2-hexadecen-1-ol | Terpenoid | C20H40O | 296 | 5.49 |
| 8 | 13.95 | (Z)-methyl hexadec-11-enoate | Fatty acid methyl ester | C17H32O2 | 268 | 1.64 |
| 9 | 14.26 | Hexadecanoic acid methyl ester | Fatty acid methyl ester | C17H34O2 | 270 | 12.27 |
| 10 | 14.91 | n-hexadecanoic acid | Fatty acid | C16H32O2 | 256 | 4.02 |
| 11 | 17 | 7,10-octadecadienoic acid, methyl ester | Fatty acid methyl ester | C19H34O2 | 294 | 1.52 |
| 12 | 17.1 | Trans-13-octadecenoic acid, methyl ester | Fatty acid methyl ester | C19H36O2 | 296 | 13.48 |
| 13 | 17.19 | 11-octadecenoic acid, methyl ester | Fatty acid methyl ester | C19H36O2 | 296 | 1.39 |
| 14 | 17.52 | Methyl stearate | Saturated methyl ester | C19H38O2 | 298 | 8.67 |
| 15 | 17.82 | Trans-13-octadecenoic acid | Fatty acid | C18H34O2 | 282 | 0.57 |
| 16 | 19.57 | Ethyl iso-allocholate | Steroid | C26H44O5 | 436 | 24.24 |
| 17 | 20.34 | 9-hexadecenoic acid | Fatty acid | C16H30O2 | 254 | 0.5 |
| 18 | 24.3 | Phthalic acid, di(2-propylpentyl) ester | Lipophilic chemicals | C24H38O4 | 390 | 9.24 |
| 19 | 37.2 | Spirost-8-en-11-one, 3-hydroxy-, (3β,5a,14β,20β,22β,25R)- | Steroid | C27H40O4 | 428 | 1.86 |
| Tricleocarpa cylindrica | ||||||
| No. | Ret. Time (min) | Compound Name | Compound Classification | Chemical Formula | Mol. Weight | Peak Area (%) |
| 1 | 11.4 | Tetradecane, 2,6,10-trimethyl- | Terpenoid | C17H36 | 240 | 0.25 |
| 2 | 12.16 | Tetradecanoic acid | Fatty acid | C14H28O2 | 228 | 2.44 |
| 3 | 13.03 | Neophytadiene | Diterpene | C20H38 | 278 | 3.06 |
| 4 | 13.36 | 3,7,11,15-tetramethyl-2-hexadecen-1-ol | Terpenoid | C20H40O | 296 | 1.82 |
| 5 | 14.25 | Hexadecanoic acid, methyl ester | Fatty acid | C17H34O2 | 270 | 1.11 |
| 6 | 14.63 | Palmitoleic acid | Fatty acid | C16H30O2 | 254 | 1.64 |
| 7 | 14.95 | n-hexadecanoic acid | Fatty acid | C16H32O2 | 256 | 13.44 |
| 8 | 15.52 | cis-13-eicosenoic acid | Fatty acid | C20H38O2 | 310 | 0.19 |
| 9 | 15.8 | ethanol 2-(9-octadecenyloxy)-,(Z)- | Dialkyl ether | C20H40O2 | 312 | 0.39 |
| 10 | 16.07 | 10-heptadecen-8-ynoic acid, methyl ester, (E)- | Fatty acid methyl ester | C18H30O2 | 278 | 0.46 |
| 11 | 17 | 9,12-octadecadienoic acid, methyl ester, (E,E)- | Fatty acid methyl ester | C19H34O2 | 294 | 0.46 |
| 12 | 17.09 | 10-octadecenoic acid, methyl ester | Fatty acid methyl ester | C19H36O2 | 296 | 1.04 |
| 13 | 17.51 | 11-octadecenoic acid, methyl ester | Fatty acid methyl ester | C19H36O2 | 296 | 1.73 |
| 14 | 17.83 | cis-13-octadecenoic acid | Fatty acid | C18H34O2 | 282 | 1.39 |
| 15 | 19.05 | 9-hexadecenoic acid | Fatty acid | C16H30O2 | 254 | 0.5 |
| 16 | 19.57 | trans-13-octadecenoic acid | Fatty acid | C18H34O2 | 282 | 0.2 |
| 17 | 20.11 | Estra-1,3,5(10)-trien-17-β-ol-17-α-butadinyl-3-methoxy | Steroid | C18H24O | 256 | 0.43 |
| 18 | 22.55 | Ethyl iso-allocholate | Steroid | C26H44O5 | 436 | 14.67 |
| 19 | 23.17 | cis-13-eicosenoic acid | Fatty acid | C20H38O2 | 310 | 0.22 |
| 20 | 24.29 | Diisooctyl phthalate | Phtalate ester | C24H38O4 | 390 | 2.84 |
| 21 | 32.73 | 17-(1,5-dimethylhexyl)- 10,13-dimethyl 2,3,4,7,8,9,10,11,12, 13,14,15,16,17- tetradecahydro- 1H- cyclopenta[a] phenanthren-3-ol | Steroid | C27H46O | 386 | 15.15 |
| 22 | 36.89 | Stigmasta-5,24(28)-dien-3-ol, (3β,24Z)- | Steroid | C29H48O | 412 | 36.59 |
| Ulva lactuca | ||||||
| No. | Ret. Time (min) | Compound Name | Compound Classification | Chemical Formula | Mol. Weight | Peak Area (%) |
| 1 | 11.8 | 8-Heptadecene | Fatty alcohol | C17H34 | 238 | 4.93 |
| 2 | 13.03 | Neophytadiene | Terpenoid | C20H38 | 278 | 4.93 |
| 3 | 13.36 | Ethanol, 2-(9-octadecenyloxy)-, (Z)- | Dialkyl ether | C20H40O2 | 312 | 0.69 |
| 4 | 13.62 | 3,7,11,15-tetramethyl-2-hexadecen-1-ol | Terpenoid | C20H40O | 296 | 1.15 |
| 5 | 13.95 | (Z)-methyl hexadec-11-enoate | Fatty acid methyl ester | C17H32O2 | 268 | 0.89 |
| 6 | 14.26 | Hexadecanoic acid, methyl ester | Fatty acid methyl ester | C17H34O2 | 270 | 12.58 |
| 7 | 14.63 | 9-hexadecenoic acid | Fatty acid | C16H30O2 | 254 | 1.48 |
| 8 | 14.91 | n-hexadecanoic acid | Fatty acid | C16H32O2 | 256 | 5.23 |
| 9 | 16.99 | 8,11-octadecadienoic acid, methyl ester | Fatty acid methyl ester | C19H34O2 | 294 | 3.01 |
| 10 | 17.09 | trans-13-octadecenoic acid, methyl ester | Fatty acid methyl ester | C19H36O2 | 296 | 10.78 |
| 11 | 17.51 | Methyl stearate | Fatty acid methyl ester | C19H38O2 | 298 | 7.12 |
| 12 | 17.87 | trans-13-octadecenoic acid | Fatty acid | C18H34O2 | 282 | 2.21 |
| 13 | 18.7 | 7-methyl-Z-tetradecen-1-ol acetate | Ester | C17H32O2 | 268 | 13.34 |
| 14 | 24.29 | Diisooctyl phthalate | Benzoic acid ester | C24H38O4 | 390 | 9.37 |
| 15 | 27.71 | Ethyl iso-allocholate | Steroid | C26H44O5 | 436 | 4.87 |
| 16 | 30.65 | Oleic acid, 3-(octadecyloxy)propyl ester | Fatty alcohol | C39H76O3 | 592 | 1.34 |
| 17 | 37.33 | Cholest-5-en-3-ol, 24-propylidene-, (3fl)- | Steroid | C30H50O | 426 | 18.17 |
| Compound | Physiochemical Properties of Compounds Based on the Lipinski Rule | ||||
|---|---|---|---|---|---|
| Molecular Weight (Dalton) | Hydrogen Bond Donors | Hydrogen Bond Acceptors | Log P | Molar Refractivity (g/mol) | |
| <500 | ≤10 | <10 | ≤5 | 40–130 | |
| Compound1 | 334.45 | 1 | 2 | 4.47 | 101.09 |
| Compound2 | 334.45 | 1 | 2 | 6.34 | 123.61 |
| Compound3 | 412.69 | 1 | 1 | 6.62 | 132.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sunarwidhi, A.L.; Rahmaniar, W.; Prasedya, E.S.; Padmi, H.; Widyastuti, S.; Pangestu, K.W.J.; Ilhami, B.T.K.; Handayani, E.; Utami, N.W.P.; Maulana, F.A.; et al. In Vitro Anti-Oxidant, In Vivo Anti-Hyperglycemic, and Untargeted Metabolomics-Aided-In Silico Screening of Macroalgae Lipophilic Extracts for Anti-Diabetes Mellitus and Anti-COVID-19 Potential Metabolites. Metabolites 2023, 13, 1177. https://doi.org/10.3390/metabo13121177
Sunarwidhi AL, Rahmaniar W, Prasedya ES, Padmi H, Widyastuti S, Pangestu KWJ, Ilhami BTK, Handayani E, Utami NWP, Maulana FA, et al. In Vitro Anti-Oxidant, In Vivo Anti-Hyperglycemic, and Untargeted Metabolomics-Aided-In Silico Screening of Macroalgae Lipophilic Extracts for Anti-Diabetes Mellitus and Anti-COVID-19 Potential Metabolites. Metabolites. 2023; 13(12):1177. https://doi.org/10.3390/metabo13121177
Chicago/Turabian StyleSunarwidhi, Anggit Listyacahyani, Wahyu Rahmaniar, Eka Sunarwidhi Prasedya, Hasriaton Padmi, Sri Widyastuti, Kukuh Waseso Jati Pangestu, Bq Tri Khairina Ilhami, Ervina Handayani, Ni Wayan Putri Utami, Farreh Alan Maulana, and et al. 2023. "In Vitro Anti-Oxidant, In Vivo Anti-Hyperglycemic, and Untargeted Metabolomics-Aided-In Silico Screening of Macroalgae Lipophilic Extracts for Anti-Diabetes Mellitus and Anti-COVID-19 Potential Metabolites" Metabolites 13, no. 12: 1177. https://doi.org/10.3390/metabo13121177
APA StyleSunarwidhi, A. L., Rahmaniar, W., Prasedya, E. S., Padmi, H., Widyastuti, S., Pangestu, K. W. J., Ilhami, B. T. K., Handayani, E., Utami, N. W. P., Maulana, F. A., Ichfa, M. S. M., & Hernawan, A. (2023). In Vitro Anti-Oxidant, In Vivo Anti-Hyperglycemic, and Untargeted Metabolomics-Aided-In Silico Screening of Macroalgae Lipophilic Extracts for Anti-Diabetes Mellitus and Anti-COVID-19 Potential Metabolites. Metabolites, 13(12), 1177. https://doi.org/10.3390/metabo13121177