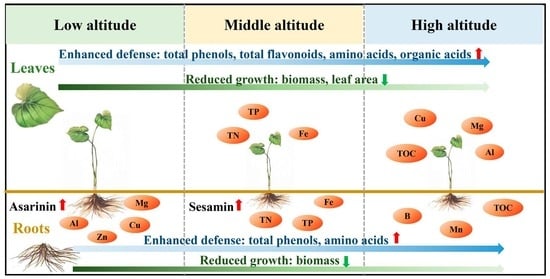
Altitudinal Variation on Metabolites, Elements, and Antioxidant Activities of Medicinal Plant Asarum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Growth Condition and Plant Materials
2.2. Determination of Nutrient Element and Soil Properties
2.3. Determination of Growth and Physiological Indices
2.3.1. Growth Indices Measurement
2.3.2. Extraction and HPLC Analysis of Asarinin and Sesamin
2.3.3. Total Phenolic and Total Flavonoid Contents
2.3.4. Antioxidant Activity
2.4. Metabolite Extraction and Detection
2.4.1. Primary Metabolite Analysis
2.4.2. Phenol Metabolites Analysis
2.5. Statistical Analysis
3. Results
3.1. Soil Chemical Properties
3.2. Nutritional Element Distribution and Physiological Indices in Asarum at Different Altitudes
3.3. Metabolic Profile in Asarum at Different Altitudes
3.4. Correlations of Metabolites–Elements–Medicinal Ingredients in Asarum
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Devadoss, J.; Falco, N.; Dafflon, B.; Wu, Y.X.; Franklin, M.; Hermes, A.; Hinckley, E.L.S.; Wainwright, H. Remote Sensing-Informed Zonation for Understanding Snow, Plant and Soil Moisture Dynamics within a Mountain Ecosystem. Remote Sens. 2020, 12, 2733. [Google Scholar] [CrossRef]
- Gao, M.D.; Wang, X.H.; Meng, F.D.; Liu, Q.; Li, X.Y.; Zhang, Y.; Piao, S.L. Three-dimensional change in temperature sensitivity of northern vegetation phenology. Glob. Change Biol. 2020, 26, 5189–5201. [Google Scholar] [CrossRef] [PubMed]
- Read, Q.D.; Moorhead, L.C.; Swenson, N.G.; Bailey, J.K.; Sanders, N.J. Convergent effects of elevation on functional leaf traits within and among species. Funct. Ecol. 2014, 28, 37–45. [Google Scholar] [CrossRef]
- Rahman, I.U.; Afzal, A.; Iqbal, Z.; Hart, R.; Abd Allah, E.F.; Alqarawi, A.A.; Alsubeie, M.S.; Calixto, E.S.; Ijaz, F.; Ali, N.; et al. Response of plant physiological attributes to altitudinal gradient: Plant adaptation to temperature variation in the Himalayan region. Sci. Total Environ. 2020, 706, 135714. [Google Scholar] [CrossRef]
- Jahdi, R.; Arabi, M.; Bussotti, F. Effect of environmental gradients on leaf morphological traits in the Fandoghlo forest region (NW Iran). Iforest 2020, 13, 523–530. [Google Scholar] [CrossRef]
- Hashim, A.M.; Alharbi, B.M.; Abdulmajeed, A.M.; Elkelish, A.; Hozzein, W.N.; Hassan, H.M. Oxidative stress responses of some endemic plants to high altitudes by intensifying antioxidants and secondary metabolites content. Plants. 2020, 9, 869. [Google Scholar] [CrossRef]
- Golob, A.; Luzar, N.; Kreft, I.; Germ, M. Adaptative Responses of Common and Tartary Buckwheat to Different Altitudes. Plants 2022, 11, 1439. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Kong, D.X.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.C.; Zhang, L.; Zou, H.Y.; Qiu, L.; Zheng, Y.W.; Yang, D.F.; Wang, Y.P. Effects of Light on Secondary Metabolite Biosynthesis in Medicinal Plants. Front. Plant Sci. 2021, 12, 781236. [Google Scholar] [CrossRef] [PubMed]
- Kfoury, N.; Scott, E.R.; Orians, C.M.; Ahmed, S.; Cash, S.B.; Griffin, T.; Matyas, C.; Stepp, J.R.; Han, W.Y.; Xue, D.Y.; et al. Plant-Climate Interaction Effects: Changes in the Relative Distribution and Concentration of the Volatile Tea Leaf Metabolome in 2014–2016. Front. Plant Sci. 2019, 10, 1518. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, F.L.; Liu, J.; Guan, F.C.; Quan, H.; Meng, F.J. The adaptation strategies of Herpetospermum pedunculosum (Ser.) Baill at altitude gradient of the Tibetan plateau by physiological and metabolomic methods. BMC Genom. 2019, 20, 451. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Joshi, R.; Kumar, R. Metabolic signatures provide novel insights to Picrorhiza kurroa adaptation along the altitude in Himalayan region. Metabolomics 2020, 16, 77. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Singh, V.K.; Lee, Y.; Kumar, S.; Rai, P.K.; Pathak, A.K.; Singh, V.K. Analysis of Mineral Elements in Medicinal Plant Samples Using LIBS and ICP-OES. Atom. Spectrosc. 2020, 41, 234–241. [Google Scholar] [CrossRef]
- Song, W.Y.; Zhang, Z.B.; Shao, H.B.; Guo, X.L.; Cao, H.X.; Zhao, H.B.; Fu, Z.Y.; Hu, X.J. Relationship between calcium decoding elements and plant abiotic-stress resistance. Int. J. Biol. Sci. 2008, 4, 116–125. [Google Scholar] [CrossRef]
- Singh, D.P.; Liu, L.H.; Oiseth, S.K.; Beloy, J.; Lundin, L.; Gidley, M.J.; Day, L. Influence of Boron on Carrot Cell Wall Structure and Its Resistance to Fracture. J. Agr. Food Chem. 2010, 58, 9181–9189. [Google Scholar] [CrossRef]
- Langer, S.E.; Marina, M.; Burgos, J.L.; Martínez, G.A.; Civello, P.M.; Villarreal, N.M. Calcium chloride treatment modifies cell wall metabolism and activates defense responses in strawberry fruit (Fragaria × ananassa, Duch). J. Sci. Food Agr. 2019, 99, 4003–4010. [Google Scholar] [CrossRef] [PubMed]
- Kelly, L.M.; González, F. Phylogenetic relationships in Aristolochiaceae. Syst. Bot. 2003, 28, 236–249. [Google Scholar]
- Liu, H.Z.; Wang, C.H. The genus: A review on phytochemistry, ethnopharmacology, toxicology and pharmacokinetics. J. Ethnopharmacol. 2022, 282, 114642. [Google Scholar] [CrossRef]
- Chen, C.; Shi, X.W.; Zhou, T.; Li, W.M.; Li, S.F.; Bai, G.Q. Full-length transcriptome analysis and identification of genes involved in asarinin and aristolochic acid biosynthesis in medicinal plant. Genome 2021, 64, 639–653. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Commission. Chinese Pharmacopoeia Part I; China Medical Science Press: Beijing, China, 2020. [Google Scholar]
- Jeong, M.; Kim, H.M.; Lee, J.S.; Choi, J.H.; Jang, D.S. (-)-Asarinin from the Roots of Induces Apoptotic Cell Death via Caspase Activation in Human Ovarian Cancer Cells. Molecules 2018, 23, 1849. [Google Scholar] [CrossRef]
- Liu, F.W.; Ali, T.; Liu, Z. Comparative Transcriptomic Analysis Reveals the Effects of Drought on the Biosynthesis of Methyleugenol in Asarum sieboldii Miq. Biomolecules 2021, 11, 1233. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhao, J.; Pan, F.J.; Li, D.J.; Chen, H.S.; Wang, K.L. Changes in nitrogen and phosphorus limitation during secondary succession in a karst region in southwest China. Plant Soil. 2015, 391, 77–91. [Google Scholar] [CrossRef]
- Hur, S.H.; Kim, H.; Kim, Y.K.; Lee, J.H.; Na, T.; Baek, E.J.; Kim, H.J. Simultaneous quantification of 60 elements associated with dried red peppers by ICP for routine analysis. J. Food Meas. Charact. 2023, 17, 5185–5194. [Google Scholar] [CrossRef]
- Abramovic, H.; Grobin, B.; Ulrih, N.P.; Cigic, B. Relevance and Standardization of In Vitro Antioxidant Assays: ABTS, DPPH, and Folin-Ciocalteu. J. Chem. 2018, 2018, 4608405. [Google Scholar] [CrossRef]
- Sakanaka, S.; Tachibana, Y.; Okada, Y. Preparation and antioxidant properties of extracts of Japanese persimmon leaf tea (kakinoha-cha). Food Chem. 2005, 89, 569–575. [Google Scholar] [CrossRef]
- Szabo, M.R.; Iditoiu, C.; Chambre, D.; Lupea, A.X. Improved DPPH determination for antioxidant activity spectrophotometric assay. Chem. Pap. 2007, 61, 214–216. [Google Scholar] [CrossRef]
- Dong, J.W.; Cai, L.; Xing, Y.; Yu, J.; Ding, Z.T. Re-evaluation of ABTS center dot plus Assay for Total Antioxidant Capacity of Natural Products. Nat. Prod. Commun. 2015, 10, 2169–2172. [Google Scholar]
- Pavlopoulos, G.A.; O’Donoghue, S.I.; Satagopam, V.P.; Soldatos, T.G.; Pafilis, E.; Schneider, R. Arena3D: Visualization of biological networks in 3D. Bmc Syst. Biol. 2008, 2, 104. [Google Scholar] [CrossRef]
- Jiang, Y.A.; Cao, Y.T.; Han, S.J.; Zhang, J.H.; Hao, L. Spatial Variation and Temporal Instability in the Growth/Climate Relationship of White Birch (Betula platyphylla Suk) in the Changbai Mountain, China. Forests 2021, 12, 589. [Google Scholar] [CrossRef]
- Ahmad, K.S.; Hameed, M.; Deng, J.B.; Ashraf, M.; Hamid, A.; Ahmad, F.; Fatima, S.; Akhtar, N. Ecotypic adaptations in Bermuda grass (Cynodon dactylon) for altitudinal stress tolerance. Biologia 2016, 71, 885–895. [Google Scholar] [CrossRef]
- Johnstone, J. Alpine Plant Life: Functional Plant Ecology of High Mountain Ecosystems. Mt. Res. Dev. 2021, 41, M1–M2. [Google Scholar] [CrossRef]
- Tian, M.; Yu, G.R.; He, N.P.; Hou, J.H. Leaf morphological and anatomical traits from tropical to temperate coniferous forests: Mechanisms and influencing factors. Sci. Rep. 2016, 6, 19703. [Google Scholar] [CrossRef]
- Ullah, R.; Khan, N.; Ali, K. Which factor explains the life-history of Xanthium strumarium L., an aggressive alien invasive plant species, along its altitudinal gradient? Plant Direct 2022, 6, e375. [Google Scholar] [CrossRef]
- Basnett, S.; Devy, S.M. Phenology determines leaf functional traits across Rhododendron species in the Sikkim Himalaya. Alp. Bot. 2021, 131, 63–72. [Google Scholar] [CrossRef]
- Cairns, D.M. Alpine Treelines: Functional Ecology of The Global High Elevation Tree Limits (vol 45, pg 421, 2013). Arct. Antarct. Alp. Res. 2014, 46, 292. [Google Scholar] [CrossRef]
- Ahmad, K.S.; Qureshi, R.; Hameed, M.; Ahmad, F.; Nawaz, T. Conservation Assessment and Medicinal Importance of some Plants Resources from Sharda, Neelum Valley, Azad Jammu and Kashmir, Pakistan. Int. J. Agric. Biol. 2012, 14, 997–1000. [Google Scholar]
- Bucher, S.F.; Römermann, C. The timing of leaf senescence relates to flowering phenology and functional traits in 17 herbaceous species along elevational gradients. J. Ecol. 2021, 109, 1537–1548. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, C.X.; Zhao, X.H.; Tan, Q.L.; Sun, X.C.; Cao, A.Y.; Cui, M.; Zhang, Y. Molybdenum improves antioxidant and osmotic-adjustment ability against salt stress in Chinese cabbage. Plant Soil. 2012, 355, 375–383. [Google Scholar] [CrossRef]
- Nazir, F.; Hussain, A.; Fariduddin, Q. Hydrogen peroxide modulate photosynthesis and antioxidant systems in tomato (Solanum lycopersicum L.) plants under copper stress. Chemosphere 2019, 230, 544–558. [Google Scholar] [CrossRef]
- Riaz, M.; Kamran, M.; El-Esawi, M.A.; Hussain, S.; Wang, X.R. Boron-toxicity induced changes in cell wall components, boron forms, and antioxidant defense system in rice seedlings. Ecotoxicol. Environ. Saf. 2021, 216, 112192. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, S.; Zehra, A.; Mukarram, M.; Wani, K.I.; Naeem, M.; Khan, M.M.A.; Aftab, T. Salicylic acid-mediated alleviation of soil boron toxicity in and: Growth, antioxidant responses, essential oil contents and components. Chemosphere 2021, 276, 130153. [Google Scholar] [CrossRef]
- Rizhsky, L.; Liang, H.J.; Shuman, J.; Shulaev, V.; Davletova, S.; Mittler, R. When Defense pathways collide. The response of Arabidopsis to a combination of drought and heat stress. Plant Physiol. 2004, 134, 1683–1696. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Sun, X.D.; Kong, X.X.; Galvan, J.V.; Li, X.; Yang, S.H.; Yang, Y.Q.; Yang, Y.P.; Hu, X.Y. Physiological, biochemical and proteomics analysis reveals the adaptation strategies of the alpine plant Potentilla saundersiana at altitude gradient of the Northwestern Tibetan Plateau. J. Proteom. 2015, 112, 63–82. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Xie, Y.Y.; Li, W.Q.; Wei, M.H.; Dai, T.; Li, Z.; Wang, B.Z. Physiological and Transcriptomic Analyses Reveal Exogenous Trehalose Is Involved in the Responses of Wheat Roots to High Temperature Stress. Plants 2021, 10, 2644. [Google Scholar] [CrossRef]
- Vigani, G.; Bashir, K.; Ishimaru, Y.; Lehmann, M.; Casiraghi, F.M.; Nakanishi, H.; Seki, M.; Geigenberger, P.; Zocchi, G.; Nishizawa, N.K. Knocking down mitochondrial iron transporter (MIT) reprograms primary and secondary metabolism in rice plants. J. Exp. Bot. 2016, 67, 1357–1368. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, H.; Tadayon, M.R.; Nadeem, M.; Cheema, M.; Razmjoo, J. Proline-mediated changes in antioxidant enzymatic activities and the physiology of sugar beet under drought stress. Acta Physiol. Plant 2019, 41, 23. [Google Scholar] [CrossRef]
- Ostersetzer-Biran, O.; Klipcan, L. Aminoacyl-tRNA synthetases and translational quality control in plant mitochondria. Mitochondrion 2020, 54, 15–20. [Google Scholar] [CrossRef]
- Cai, J.H.; Aharoni, A. Amino acids and their derivatives mediating defense priming and growth tradeoff. Curr. Opin. Plant Biol. 2022, 69, 102288. [Google Scholar] [CrossRef]
- Kumar, R.; Joshi, R.; Kumari, M.; Thakur, R.; Kumar, D.; Kumar, S. Elevated CO2 and temperature influence key proteins and metabolites associated with photosynthesis, antioxidant and carbon metabolism in Picrorhiza kurroa. J. Proteom. 2020, 219, 103755. [Google Scholar] [CrossRef]
- Kumar, P.; Acharya, V.; Warghat, A.R. Comparative transcriptome analysis infers bulb derived in vitro cultures as a promising source for sipeimine biosynthesis in Fritillaria cirrhosa D. Don (Liliaceae, syn. Fritillaria roylei Hook.)—High value Himalayan medicinal herb. Phytochemistry 2021, 183, 112631. [Google Scholar] [CrossRef]
- Joshi, R.; Kumar, R. Unique Metabolic Shift Reveals Potential Mechanism of Cold and Freezing Acclimatization. J. Plant Growth Regul. 2023, 42, 5763–5779. [Google Scholar] [CrossRef]
- Adhikari, P.; Joshi, K.; Singh, M.; Pandey, A. Influence of altitude on secondary metabolites, antioxidants, and antimicrobial activities of Himalayan yew (Taxus wallichiana). Plant Biosyst. 2022, 156, 187–195. [Google Scholar] [CrossRef]
- Nasri, Z.; Ahmadi, M.; Striesow, J.; Ravandeh, M.; von Woedtke, T.; Wende, K. Insight into the Impact of Oxidative Stress on the Barrier Properties of Lipid Bilayer Models. Int. J. Mol. Sci. 2022, 23, 5932. [Google Scholar] [CrossRef] [PubMed]
- Jafri, S.A.A.; Khalid, Z.M.; Khan, M.Z.; Jogezai, N. Evaluation of phytochemical and antioxidant potential of various extracts from traditionally used medicinal plants of Pakistan. Open Chem. 2022, 20, 1337–1356. [Google Scholar] [CrossRef]
- Joshi, K.; Adhikari, P.; Bhatt, I.D.; Pande, V. Source dependent variation in phenolics, antioxidant and antimicrobial activity of Paeonia emodi in west Himalaya, India. Physiol. Mol. Biol. Plants 2022, 28, 1785–1798. [Google Scholar] [CrossRef]
- Moreira-Vilar, F.C.; da Silva, H.A.; Marchiosi, R.; Siqueira-Soares, R.D.; Finger-Teixeira, A.; da Silva, K.G.; Contesoto, I.D.; Constantin, R.P.; dos Santos, W.D.; Ferrarese-Filho, O. p-Methoxycinnamic acid disturbs cellular respiration and increases the lignification of roots. Plant Biosyst. 2023, 157, 12–23. [Google Scholar] [CrossRef]
- Zdunska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant Properties of Ferulic Acid and Its Possible Application. Skin. Pharmacol. Phys. 2018, 31, 332–336. [Google Scholar] [CrossRef]
- Vuletic, M.; Sukalovic, V.H.T.; Markovic, K.; Kravic, N.; Vucinic, Z.; Maksimovic, V. Differential response of antioxidative systems of maize (Zea mays L.) roots cell walls to osmotic and heavy metal stress. Plant Biol. 2014, 16, 88–96. [Google Scholar] [CrossRef]
- Dong, H.; Li, M.L.; Jin, L.; Xie, X.R.; Li, M.F.; Wei, J.H. Cool Temperature Enhances Growth, Ferulic Acid and Flavonoid Biosynthesis While Inhibiting Polysaccharide Biosynthesis in Angelica sinensis. Molecules 2022, 27, 320. [Google Scholar] [CrossRef]
- Murathan, Z.T. Comparison of the Bioactive and Nutrient Profiles of Persimmon Fruits Grown Under Different Ecological Conditions. Erwerbs-Obstbau 2023, 65, 539–546. [Google Scholar] [CrossRef]
- Guo, J.X.; Lu, X.Y.; Tao, Y.F.; Guo, H.J.; Min, W. Comparative Ionomics and Metabolic Responses and Adaptive Strategies of Cotton to Salt and Alkali Stress. Front. Plant Sci. 2022, 13, 871387. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.J.; Huang, Y.X.; Zhou, H.J.; Adeleye, A.S.; Wang, H.T.; Ortiz, C.; Mazer, S.J.; Keller, A.A. GC-TOF-MS based metabolomics and ICP-MS based metallomics of cucumber fruits reveal alteration of metabolites profile and biological pathway disruption induced by nano copper. Env. Sci. Nano 2016, 3, 1114–1123. [Google Scholar] [CrossRef]
- Zhao, R.; Ren, W.J.; Wang, H.M.; Li, Z.X.; Teng, Y.; Luo, Y.M. Nontargeted metabolomic analysis to unravel alleviation mechanisms of carbon nanotubes on inhibition of alfalfa growth under pyrene stress. Sci. Total Environ. 2022, 852, 158405. [Google Scholar] [CrossRef] [PubMed]
- Trouvelot, S.; Héloir, M.C.; Poinssot, B.; Gauthier, A.; Paris, F.; Guillier, C.; Combier, M.; Trdá, L.; Daire, X.; Adrian, M. Carbohydrates in plant immunity and plant protection: Roles and potential application as foliar sprays. Front. Plant Sci. 2014, 5, 592. [Google Scholar] [CrossRef]
- Williamson, G.; Kay, C.D.; Crozier, A. The Bioavailability, Transport, and Bioactivity of Dietary Flavonoids: A Review from a Historical Perspective. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1054–1112. [Google Scholar] [CrossRef]
- Ray, S.; Mishra, S.; Bisen, K.; Singh, S.; Sarma, B.K.; Singh, H.B. Modulation in phenolic root exudate profile of Abelmoschus esculentus expressing activation of defense pathway. Microbiol. Res. 2018, 207, 100–107. [Google Scholar] [CrossRef] [PubMed]







| Site | Vegetation Type | Elevation (m) | Longitude and Latitude | Aspect | MAT (°C) | MAP (mm) | MASR (kJ m−2 day−1) | Dominant Species |
|---|---|---|---|---|---|---|---|---|
| 1 | Deciduous broad-leaved forest | 620.79– 625.50 | 126°6′37.67″ N 41°53′54.10″ E | East | 3.58 | 894.00 | 6907.00 | Erythronium japonicum Decne., Polygonatum acuminatifolium Kom., Hylomecon japonica (Thunb.) Prantl & Kündig, Cardamine leucantha (Tausch) O. E. Schulz, Meehania urticifolia (Miq.) Makino |
| 2 | Deciduous broad-leaved forest | 827.60– 834.94 | 126°5′25.39″ N 41°55′50.56″ E | West | 2.80 | 911.00 | 6941.00 | Erythronium japonicum Decne., Anemone amurensis (Korsh.) Kom., Anemone raddeana Regel, Hylomecon japonica (Thunb.) Prantl & Kündig, Lilium distichum Nakai ex Kamibayashi |
| 3 | Deciduous broad-leaved forest | 1020.37– 1022.74 | 126°4′39.67″ N 41°56′1.46″ E | Southeast | 2.97 | 897.00 | 7263.00 | Gymnospermium microrrhynchum (S. Moore) Takht., Erythronium japonicum Decne., Maianthemum japonicum (A. Gray) LaFrankie, Anemone amurensis (Korsh.) Kom., Anemone raddeana Regel |
| LOD (mg/L) | L (Low Altitude) | M (Middle Altitude) | H (High Altitude) | |
|---|---|---|---|---|
| pH | - | 6.19 ± 0.18 a | 5.77 ± 0.16 a | 5.62 ± 0.46 a |
| EC (ms/cm) | - | 1.76 ± 72.99 a | 1.32 ± 17.58 a | 1.63 ± 11.02 a |
| TOC (%) | - | 6.84 ± 4.53 c | 8.88 ± 5.14 b | 11.24 ± 1.55 a |
| TN (%) | - | 0.93 ± 0.04 ab | 0.81 ± 0.70 b | 1.16 ± 0.18 a |
| TP (%) | - | 0.08 ± 0.05 b | 0.1 ± 0.03 a | 0.06 ± 0.02 c |
| Mg (mg/kg) | 0.0001 | 4104.79 ± 148.61 b | 4948.05 ± 79.02 a | 4151.74 ± 62.78 b |
| Al (mg/kg) | 0.0001 | 33,945.73 ± 1902.79 c | 39,451.17 ± 863.20 b | 53,979.69 ± 2747.76 a |
| Fe (mg/kg) | 0.0005 | 20,733.57 ± 1464.33 b | 18,692.84 ± 182.27 c | 25,240 ± 519.97 a |
| Zn (mg/kg) | 0.0004 | 78.59 ± 4.03 b | 82.71 ± 3.56 b | 123.56 ± 11.24 a |
| Mn (mg/kg) | 0.0002 | 552.36 ± 53.24 b | 534.11 ± 9.02 b | 850.42 ± 29.29 a |
| Cu (mg/kg) | 0.001 | 11.22 ± 0.84 c | 13.09 ± 0.46 b | 16.4 ± 0.76 a |
| B (mg/kg) | 0.0014 | 35.05 ± 1.08 a | 27.27 ± 0.35 b | 27.6 ± 2.35 b |
| Mo (mg/kg) | 0.0001 | 0.53 ± 0.06 c | 0.63 ± 0.01 b | 0.79 ± 0.06 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, L.; Yang, N.; Sui, Y.; Li, Y.; Zhao, W.; Zhang, L.; Mu, L.; Tang, Z. Altitudinal Variation on Metabolites, Elements, and Antioxidant Activities of Medicinal Plant Asarum. Metabolites 2023, 13, 1193. https://doi.org/10.3390/metabo13121193
Pan L, Yang N, Sui Y, Li Y, Zhao W, Zhang L, Mu L, Tang Z. Altitudinal Variation on Metabolites, Elements, and Antioxidant Activities of Medicinal Plant Asarum. Metabolites. 2023; 13(12):1193. https://doi.org/10.3390/metabo13121193
Chicago/Turabian StylePan, Liben, Nan Yang, Yushu Sui, Yi Li, Wen Zhao, Liqiu Zhang, Liqiang Mu, and Zhonghua Tang. 2023. "Altitudinal Variation on Metabolites, Elements, and Antioxidant Activities of Medicinal Plant Asarum" Metabolites 13, no. 12: 1193. https://doi.org/10.3390/metabo13121193
APA StylePan, L., Yang, N., Sui, Y., Li, Y., Zhao, W., Zhang, L., Mu, L., & Tang, Z. (2023). Altitudinal Variation on Metabolites, Elements, and Antioxidant Activities of Medicinal Plant Asarum. Metabolites, 13(12), 1193. https://doi.org/10.3390/metabo13121193