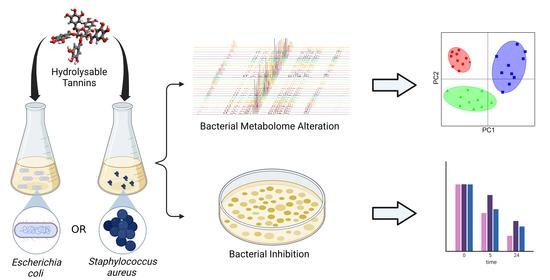
NMR Metabolomics and DNA Sequencing of Escherichia coli and Staphylococcus aureus Cultures Treated with Hydrolyzable Tannins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Bacterial Culture Conditions
2.3. Batch Culture Fermentation
2.4. Hydrolyzable Tannin Isolation and Characterization
2.5. NMR Metabolomics
2.5.1. Sample Preparation
2.5.2. Data Acquisition and Preprocessing
2.5.3. Multivariate Analysis
2.5.4. Individual Metabolite Responses
2.6. Bacterial Growth with Plate Measurements
2.7. Bacterial Culture DNA Sequencing
3. Results and Discussion
3.1. NMR Metabolomics
3.1.1. Metabolomic Alterations in E. coli Culture
3.1.2. Metabolomic Alterations in S. aureus Culture
3.1.3. Metabolomic Alterations in Fecal Batch Cultures with Ampicillin-Resistant S. aureus
3.2. Bacterial Growth Inhibition from Plate Measurements
3.3. Bacterial DNA Sequencing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A




References
- Livermore, D.M. Has the Era of Untreatable Infections Arrived? J. Antimicrob. Chemother. 2009, 64, i29–i36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Founou, L.L.; Founou, R.C.; Essack, S.Y. Antimicrobial Resistance in the Farm-to-Plate Continuum: More than a Food Safety Issue. Future Sci. OA 2021, 7, FSO692. [Google Scholar] [CrossRef]
- Liu, J.B.; Ding, Y.S.; Zhang, Y.; Chen, J.B.; Cui, B.S.; Bai, J.Y.; Lin, M.B.; Hou, Q.; Zhang, P.C.; Li, S. Anti-Inflammatory Hydrolyzable Tannins from Myricaria bracteata. J. Nat. Prod. 2015, 78, 1015–1025. [Google Scholar] [CrossRef]
- Thomas, M.L.R.M.G.; Filho, J.M.B. Anti-Inflammatory Actions of Tannins Isolated from the Bark of Anacardwm occidentale L. J. Ethnopharmacol. 1985, 13, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, G.; Nishioka, I.; Nishizawa, M.; Yamagishi, T.; Kashiwada, Y.; Dutschman, G.E.; Bodner, A.J.; Kilkuskie, R.E.; Cheng, Y.-C.; Lee, K.-H. Anti-Aids Agents, 2: Inhibitory Effect of Tannins on HIV Reverse Transcriptase and HIV Replication in H9 Lymphocyte Cells. J. Nat. Prod. 1990, 53, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, H.; Murakami, T.; Yamamoto, N.; Sakagami, H.; Tanuma, S.-I.; Hatano, T.; Yoshida, T.; Okuda, T. Inhibition of Human Immunodeficiency Viral Replication by Tannins and Related Compounds. Antivir. Res. 1992, 18, 91–103. [Google Scholar] [CrossRef]
- Corthout, J.; Pieters, L.A.; Claeys, M.; vanden Berghe, D.A.; Vlietinck, A.J. Antiviral Ellagitannins from Spondias Mombin. Phytochemistry 1991, 30, 1129–1130. [Google Scholar] [CrossRef]
- Quideau, S.; Varadinova, T.; Karagiozova, D.; Jourdes, M.; Pardon, P.; Baudry, C.; Genova, P.; Diakov, T.; Petrova, R. Main Structural and Stereochemical Aspects of the Antiherpetic Activity of Nonahydroxyterphenoyl-Containing C-Glycosidic Ellagitannins. Chem. Biodivers. 2004, 1, 247–258. [Google Scholar] [CrossRef]
- Funatogawa, K.; Hayashi, S.; Shimomura, H.; Yoshida, T.; Hatano, T.; Ito, H.; Hirai, Y. Antibacterial Activity of Hydrolyzable Tannins Derived from Medicinal Plants against Helicobacter pylori. Microbiol. Immunol. 2004, 48, 251–261. [Google Scholar] [CrossRef]
- Gontijo, D.C.; Gontijo, P.C.; Brandão, G.C.; Diaz, M.A.N.; de Oliveira, A.B.; Fietto, L.G.; Leite, J.P.V. Antioxidant Study Indicative of Antibacterial and Antimutagenic Activities of an Ellagitannin-Rich Aqueous Extract from the Leaves of Miconia latecrenata. J. Ethnopharmacol. 2019, 236, 114–123. [Google Scholar] [CrossRef]
- Karonen, M.; Ahern, J.R.; Legroux, L.; Suvanto, J.; Engström, M.T.; Sinkkonen, J.; Salminen, J.-P.; Hoste, H. Ellagitannins Inhibit the Exsheathment of Haemonchus contortus and Trichostrongylus colubriformis Larvae: The Efficiency Increases Together with the Molecular Size. J. Agric. Food Chem. 2020, 68, 4176–4186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puljula, E.; Walton, G.; Woodward, M.J.; Karonen, M. Antimicrobial Activities of Ellagitannins against Clostridiales perfringens, Escherichia coli, Lactobacillus plantarum and Staphylococcus aureus. Molecules 2020, 25, 3714. [Google Scholar] [CrossRef] [PubMed]
- Aguilera-Correa, J.J.; Fernández-López, S.; Cuñas-Figueroa, I.D.; Pérez-Rial, S.; Alakomi, H.L.; Nohynek, L.; Oksman-Caldentey, K.M.; Salminen, J.P.; Esteban, J.; Cuadros, J.; et al. Sanguiin H-6 Fractionated from Cloudberry (Rubus chamaemorus) Seeds Can Prevent the Methicillin-Resistant Staphylococcus aureus Biofilm Development during Wound Infection. Antibiotics 2021, 10, 1481. [Google Scholar] [CrossRef] [PubMed]
- Moilanen, J.; Salminen, J.-P. Ecologically Neglected Tannins and Their Biologically Relevant Activity: Chemical Structures of Plant Ellagitannins Reveal Their in Vitro Oxidative Activity at High PH. Chemoecology 2008, 18, 73–83. [Google Scholar] [CrossRef]
- Yoshida, T.; Koyama, S.; Okuda, T. Effects of the Interaction of Tannins with Co-Existing Substances. I. Inhibitory Effects of Tannins on Cupric Ion-Catalyzed Autoxidation of Ascorbic Acid. Yakugaku Zasshi 1981, 101, 695–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawamoto, H.; Nakatsubo, F.; Murakami, K. Stoichiometric Studies of Tannin-Protein Co-Precipitation. Phytochemistry 1996, 41, 1427–1431. [Google Scholar] [CrossRef] [PubMed]
- Frazier, R.A.; Papadopoulou, A.; Mueller-Harvey, I.; Kissoon, D.; Green, R.J. Probing Protein-Tannin Interactions by Isothermal Titration Microcalorimetry. J. Agric. Food Chem. 2003, 51, 5189–5195. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, B.; Salminen, J.P.; Smolander, A.; Kitunen, V. Precipitation of Proteins by Tannins: Effects of Concentration, Protein/Tannin Ratio and PH. Int. J. Food Sci. Technol. 2012, 47, 875–878. [Google Scholar] [CrossRef]
- Karonen, M.; Oraviita, M.; Mueller-Harvey, I.; Salminen, J.-P.; Green, R.J. Ellagitannins with Glucopyranose Cores Have Higher Affinities to Proteins than Acyclic Ellagitannins by Isothermal Titration Calorimetry. J. Agric. Food Chem. 2019, 67, 12730–12740. [Google Scholar] [CrossRef]
- Engström, M.T.; Virtanen, V.; Salminen, J.P. Influence of the Hydrolyzable Tannin Structure on the Characteristics of Insoluble Hydrolyzable Tannin-Protein Complexes. J. Agric. Food Chem. 2022, 70, 13036–13048. [Google Scholar] [CrossRef]
- Sekowski, S.; Veiko, A.; Olchowik-Grabarek, E.; Dubis, A.; Wilczewska, A.Z.; Markiewicz, K.H.; Zavodnik, I.B.; Lapshina, E.; Dobrzynska, I.; Abdulladjanova, N.; et al. Hydrolysable Tannins Change Physicochemical Parameters of Lipid Nano-Vesicles and Reduce DPPH Radical—Experimental Studies and Quantum Chemical Analysis. Biochim. Biophys. Acta Biomembr. 2022, 1864, 183778. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, V.; Green, R.J.; Karonen, M. Interactions between Hydrolysable Tannins and Lipid Vesicles from Escherichia coli with Isothermal Titration Calorimetry. Molecules 2022, 27, 3204. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A. Antimicrobial Properties of Tannins. Phytochemistry 1991, 30, 3875–3883. [Google Scholar] [CrossRef]
- Reo, N.V. NMR-Based Metabolomics. Drug Chem. Toxicol. 2002, 25, 375–382. [Google Scholar] [CrossRef]
- Emwas, A.-H.; Roy, R.; McKay, R.T.; Tenori, L.; Saccenti, E.; Gowda, G.A.N.; Raftery, D.; Alahmari, F.; Jaremko, L.; Jaremko, M.; et al. NMR Spectroscopy for Metabolomics Research. Metabolites 2019, 9, 123. [Google Scholar] [CrossRef] [Green Version]
- Saha, R.K.; Takahashi, T.; Kurebayashi, Y.; Fukushima, K.; Minami, A.; Kinbara, N.; Ichitani, M.; Sagesaka, Y.M.; Suzuki, T. Antiviral Effect of Strictinin on Influenza Virus Replication. Antivir. Res. 2010, 88, 10–18. [Google Scholar] [CrossRef]
- Hsieh, S.K.; Xu, J.R.; Lin, N.H.; Li, Y.C.; Chen, G.H.; Kuo, P.C.; Chen, W.Y.; Tzen, J.T.C. Antibacterial and Laxative Activities of Strictinin Isolated from Pu’er Tea (Camellia sinensis). J. Food Drug Anal. 2016, 24, 722–729. [Google Scholar] [CrossRef] [Green Version]
- ArauÌjo, A.R.; ArauÌjo, A.C.; Reis, R.L.; Pires, R.A. Vescalagin and Castalagin Present Bactericidal Activity toward Methicillin-Resistant Bacteria. ACS Biomater. Sci. Eng. 2021, 7, 1022–1030. [Google Scholar] [CrossRef]
- Liu, G.; Xiong, S.; Xiang, Y.F.; Guo, C.W.; Ge, F.; Yang, C.R.; Zhang, Y.J.; Wang, Y.F.; Kitazato, K. Antiviral Activity and Possible Mechanisms of Action of Pentagalloylglucose (PGG) against Influenza A Virus. Arch. Virol. 2011, 156, 1359–1369. [Google Scholar] [CrossRef] [Green Version]
- Kamijo, M.; Kanazawa, T.; Funaki, M.; Nishizawa, M.; Yamagishi, T. Effects of Rosa rugosa Petals on Intestinal Bacteria. Biosci Biotechnol. Biochem. 2008, 72, 773–777. [Google Scholar] [CrossRef] [PubMed]
- Salminen, J.P.; Karonen, M. Chemical Ecology of Tannins and Other Phenolics: We Need a Change in Approach. Funct. Ecol. 2011, 25, 325–338. [Google Scholar] [CrossRef]
- Mckay, R.T. How the 1D-NOESY Suppresses Solvent Signal in Metabonomics NMR Spectroscopy: An Examination of the Pulse Sequence Components and Evolution. Concepts Magn. Reson. Part A 2011, 38A, 197–220. [Google Scholar] [CrossRef]
- Jacob, D.; Deborde, C.; Lefebvre, M.; Maucourt, M.; Moing, A. NMRProcFlow: A Graphical and Interactive Tool Dedicated to 1D Spectra Processing for NMR-Based Metabolomics. Metabolomics 2017, 13, 36. [Google Scholar] [CrossRef] [Green Version]
- Emwas, A.-H.; Saccenti, E.; Gao, X.; McKay, R.T.; dos Santos, V.A.P.M.; Roy, R.; Wishart, D.S. Recommended Strategies for Spectral Processing and Post-Processing of 1D 1H-NMR Data of Biofluids with a Particular Focus on Urine. Metabolomics 2018, 14, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Meyer, T.; Sinnaeve, D.; van Gasse, B.; Tsiporkova, E.; Rietzschel, E.R.; de Buyzere, M.L.; Gillebert, T.C.; Bekaert, S.; Martins, J.C.; van Criekinge, W. NMR-Based Characterization of Metabolic Alterations in Hypertension Using an Adaptive, Intelligent Binning Algorithm. Anal. Chem. 2008, 80, 3783–3790. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. 2022. Available online: https://www.R-project.org/ (accessed on 10 November 2022).
- Allaire, J.J. RStudio: Integrated Development Environment for R; RStudio: Boston, MA, USA, 2012. [Google Scholar]
- Kucheryavskiy, S. Mdatools—R Package for Chemometrics. Chemom. Intell. Lab. Syst. 2020, 198, 103937. [Google Scholar] [CrossRef]
- Human Metabolome Database. Available online: https://hmdb.ca/ (accessed on 27 October 2022).
- Biological Magnetic Resonance Data Bank. Available online: https://bmrb.io/ (accessed on 27 October 2022).
- Wishart, D.S.; Knox, C.; Guo, A.C.; Eisner, R.; Young, N.; Gautam, B.; Hau, D.D.; Psychogios, N.; Dong, E.; Bouatra, S.; et al. HMDB: A Knowledgebase for the Human Metabolome. Nucleic Acids Res. 2009, 37, D603–D610. [Google Scholar] [CrossRef]
- Ye, Y.-F.; Zhang, L.-M.; An, Y.-P.; Hao, F.-H.; Tang, H.-R. Nuclear Magnetic Resonance for Analysis of Metabolite Composition of Escherichia coli. Chin. J. Anal. Chem. 2011, 39, 1186–1194. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, J.; Lim, Z.Y.; Aggarwal, A.; Yang, H.; Wang, S. Evaluation of the Metabolic Response of Escherichia coli to Electrolysed Water by 1H NMR Spectroscopy. LWT Food Sci. Technol. 2017, 79, 428–436. [Google Scholar] [CrossRef]
- Ye, Y.; Zhang, L.; Hao, F.; Zhang, J.; Wang, Y.; Tang, H. Global Metabolomic Responses of Escherichia coli to Heat Stress. J. Proteome Res. 2012, 11, 2559–2566. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-L.; Zhang, S.-K.; Chen, J.-Y.; Han, B.-Z. Metabolic Profiling of Staphylococcus aureus Cultivated under Aerobic and Anaerobic Conditions with 1H NMR-Based Nontargeted Analysis. Can. J. Microbiol. 2012, 58, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Ellis, R.J.; Bruce, K.D.; Jenkins, C.; Stothard, J.R.; Ajarova, L.; Mugisha, L.; Viney, M.E. Comparison of the Distal Gut Microbiota from People and Animals in Africa. PLoS ONE 2013, 8, e54783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Microbiome Helper. Available online: https://github.com/mlangill/microbiome_helper/wiki/16S-standard-operating-procedure (accessed on 24 October 2022).
- Paired-End ReAd MergeR. Available online: https://cme.h-its.org/exelixis/web/software/pear/doc.html (accessed on 24 October 2022).
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME Allows Analysis of High-Throughput Community Sequencing Data. Nat Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The StrainSelect and Greengenes Databases. Available online: https://greengenes.secondgenome.com/ (accessed on 24 October 2022).
- Engström, M.T.; Arvola, J.; Nenonen, S.; Virtanen, V.T.J.; Leppä, M.M.; Tähtinen, P.; Salminen, J.P. Structural Features of Hydrolyzable Tannins Determine Their Ability to Form Insoluble Complexes with Bovine Serum Albumin. J. Agric. Food Chem. 2019, 67, 6798–6808. [Google Scholar] [CrossRef]
- Tanaka, T.; Zhang, H.; Jiang, Z.H.; Kouno, I. Relationship between Hydrophobicity and Structure of Hydrolyzable Tannins, and Association of Tannins with Crude Drug Constituents in Aqueous Solution. Chem. Pharm. Bull. 1997, 45, 1891–1897. [Google Scholar] [CrossRef] [Green Version]
- Virtanen, V.; Karonen, M. Partition Coefficients (LogP) of Hydrolysable Tannins. Molecules 2020, 25, 3691. [Google Scholar] [CrossRef]
- Engström, M.T.; Karonen, M.; Ahern, J.R.; Baert, N.; Payré, B.; Hoste, H.; Salminen, J.P. Chemical Structures of Plant Hydrolyzable Tannins Reveal Their in Vitro Activity against Egg Hatching and Motility of Haemonchus contortus Nematodes. J. Agric. Food Chem. 2016, 64, 840–851. [Google Scholar] [CrossRef]
- Matsuo, Y.; Wakamatsu, H.; Omar, M.; Tanaka, T. Reinvestigation of the Stereochemistry of the C-Glycosidic Ellagitannins, Vescalagin and Castalagin. Org. Lett. 2015, 17, 46–49. [Google Scholar] [CrossRef]
- Beretta, G.; Artali, R.; Caneva, E.; Maffei Facino, R. Conformation of the Tridimensional Structure of 1,2,3,4,6-Pentagalloyl-β-D-Glucopyranose (PGG) by 1H NMR, NOESY and Theoretical Study and Membrane Interaction in a Simulated Phospholipid Bilayer: A First Insight. Magn. Reson. Chem. 2011, 49, 132–136. [Google Scholar] [CrossRef]
- Chen, G.H.; Lin, Y.L.; Hsu, W.L.; Hsieh, S.K.; Tzen, J.T.C. Significant Elevation of Antiviral Activity of Strictinin from Pu’er Tea after Thermal Degradation to Ellagic Acid and Gallic Acid. J. Food Drug Anal. 2015, 23, 116–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasid, N.A.; Rolfe, M.D.; Green, J.; Williamson, M.P. Homeostasis of Metabolites in Escherichia coli on Transition from Anaerobic to Aerobic Conditions and the Transient Secretion of Pyruvate. R. Soc. Open Sci. 2016, 3, 160187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okuda, T.; Hatano, T.; Ogawa, N. Rugosin D, E, F and G, Dimeric and Trimeric Hydrolyzable Tannins. Chem. Pharm. Bull. 1982, 30, 4234–4237. [Google Scholar] [CrossRef] [Green Version]
- Okuda, T.; Yoshida, T.; Hatano, T.; Iwasaki, M.; Kubo, M.; Orime, T.; Yoshizaki, M.; Naruhashi, N. Hydrolysable Tannins as Chemotaxonomic Markers in the Rosaceae. Phytochemistry 1992, 31, 3091–3096. [Google Scholar] [CrossRef]












| # and Presence in Each Set a | Metabolite | Chemical Shift b (ppm) | ||
|---|---|---|---|---|
| E. coli | S. aureus | Fecal BC | ||
| nd | nd | 1 | butyrate | 0.88 (t, 3H), 1.55 (m, 2H) |
| 2 | nd | 2 | leucine | 0.95 (t, 6H), |
| 3 | 3 | 3 | valine | 0.98 (d, 3H), 1.03 (d, 3H) |
| nd | nd | 4 | isoleucine | 1.00 (d, 3H) |
| nd | nd | 5 | propionate | 1.04 (t, 3H), 2.17 (q, 2H) |
| 6 | 6 | 6 | lactate | 1.32 (d, 3H), 4.10 (q, 1H) |
| nd | nd | 7 | alanine | 1.47 (d, 3H), 3.77 (q, 1H) |
| 8 | nd | nd | lysine | 1.73 (p, 2H), 3.03 (t, 2H) |
| 9 | 9 | 9 | acetate | 1.91 (s, 3H) |
| 10 | 10 | nd | pyruvate | 2.36 (s, 3H) |
| 11 | nd | 11 | succinate | 2.40 (s, 4H) |
| nd | nd | 12 | trimethylamine | 2.87 (s, 9H) |
| nd | nd | 13 | trimethylamine N-oxide | 3.27 (s, 9H) |
| nd | nd | 14 | glycine | 3.55 (s, 2H) |
| nd | nd | 15 | unknown c | 3.71 (m) |
| 16 | 16 | ns | glucose | 4.65 (d, 1H), 5.23 (d, 1H) |
| 17 | 17 | 17 | formate | 8.44 (s, 1H) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Virtanen, V.; Puljula, E.; Walton, G.; Woodward, M.J.; Karonen, M. NMR Metabolomics and DNA Sequencing of Escherichia coli and Staphylococcus aureus Cultures Treated with Hydrolyzable Tannins. Metabolites 2023, 13, 320. https://doi.org/10.3390/metabo13030320
Virtanen V, Puljula E, Walton G, Woodward MJ, Karonen M. NMR Metabolomics and DNA Sequencing of Escherichia coli and Staphylococcus aureus Cultures Treated with Hydrolyzable Tannins. Metabolites. 2023; 13(3):320. https://doi.org/10.3390/metabo13030320
Chicago/Turabian StyleVirtanen, Valtteri, Elina Puljula, Gemma Walton, Martin J. Woodward, and Maarit Karonen. 2023. "NMR Metabolomics and DNA Sequencing of Escherichia coli and Staphylococcus aureus Cultures Treated with Hydrolyzable Tannins" Metabolites 13, no. 3: 320. https://doi.org/10.3390/metabo13030320
APA StyleVirtanen, V., Puljula, E., Walton, G., Woodward, M. J., & Karonen, M. (2023). NMR Metabolomics and DNA Sequencing of Escherichia coli and Staphylococcus aureus Cultures Treated with Hydrolyzable Tannins. Metabolites, 13(3), 320. https://doi.org/10.3390/metabo13030320