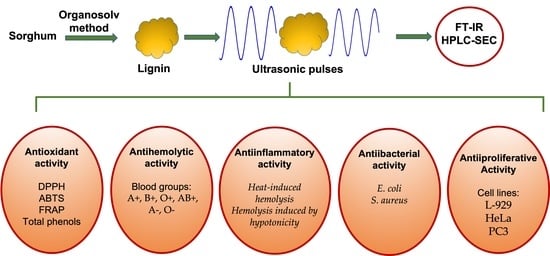
Antioxidant, Antibacterial, Anti-Inflammatory, and Antiproliferative Activity of Sorghum Lignin (Sorghum bicolor) Treated with Ultrasonic Pulses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Extraction of Lignin by the Organosolv Method
2.3. Determination of Antioxidant Activity
2.3.1. ABTS
2.3.2. DPPH
2.3.3. FRAP
2.4. Total Phenolic Content
2.5. Application of Ultrasonic Pulses to Sorghum Lignin
2.6. Determination of Antihemolytic Activity
2.7. Determination of Anti-Inflammatory Activity
2.7.1. Heat-Induced Hemolysis
2.7.2. Hemolysis Induced by Hypotonicity
2.8. Antibacterial Activity
2.9. Evaluation of Antiproliferative Activity in Cancer Cell Lines
2.10. Lignin Analysis by Fourier Transform Infrared Spectroscopy (FT-IR)
2.11. Molecular Weight Determination by Size Exclusion Chromatography (HPLC-SEC)
2.12. Statistical Analysis
3. Results and Discussion
3.1. Yield of Lignin Obtained from Sorghum Residues
3.2. Optimization of Sorghum Lignin Subjected to High-Power Ultrasonic Pulses
3.3. Antihemolytic Activity
3.3.1. Effect of Blood Groups on the Inhibition of Free Radicals Induced by AAPH Adding Sorghum Lignin with and without Pulses
3.3.2. Effect of Rh on the Inhibition of Free Radicals Induced by AAPH with Lignin with and without Pulses
3.4. Anti-Inflammatory Activity
3.4.1. Effect of Blood Groups on Anti-Inflammatory Activity by Heat in Lignin with Pulsed and Non-Ultrasonic Pulses
3.4.2. Effect of Blood Groups on Anti-Inflammatory Activity by Lignin Hypotonicity with Ultrasonic Pulses and without Ultrasonic Pulses
3.5. Antibacterial Activity
3.6. Antiproliferative Activity of Lignin on Cell Lines
3.7. Analysis by Infrared Spectroscopy with Fourier Transform (FT-IR)
3.8. Analysis by High-Performance Chromatography (HPLC)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- SIAP. Sorgo Grano. Available online: chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.gob.mx/cms/uploads/attachment/file/629688/Escenario_sorgo_mar21.pdf (accessed on 14 January 2023).
- Becker, J.; Wittmann, C. A field of dreams: Lignin valorization into chemicals, materials, fuels, and health-care products. Biotechnol. Adv. 2019, 37, 107360. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Sifontes, M.; Domine, M.E. Lignina, estructura y aplicaciones: Métodos de despolimerización para la obtención de derivados aromáticos de interés industrial. Av. En Cienc. E Ing. 2013, 4, 15–46. [Google Scholar]
- Fernández-López, J.; Lucas-González, R.; Viuda-Martos, M.; Sayas-Barberá, E.; Ballester-Sánchez, J.; Haros, C.M.; Martínez-Mayoral, A.; Pérez-Álvarez, J.A. Chemical and technological properties of bologna-type sausages with added black quinoa wet-milling coproducts as binder replacer. Food Chem. 2020, 310, 125936. [Google Scholar] [CrossRef] [PubMed]
- Bazinet, L.; Doyen, A. Antioxidants, mechanisms, and recovery by membrane processes. Crit. Rev. Food Sci. Nutr. 2017, 57, 677–700. [Google Scholar] [CrossRef] [PubMed]
- Gaspar García, J.A. Determinación de Actividad Antioxidante en Lignina Obtenida de Residuos Agrícolas. Bachelor’s Thesis, Universidad de Sonora, Hermosillo, Sonora, Mexico, 2019. [Google Scholar]
- Kosakowska, O.; Węglarz, Z.; Bączek, K. Yield and quality of ‘Greek oregano’(Origanum vulgare L. subsp. hirtum) herb from organic production system in temperate climate. Ind. Crop. Prod. 2019, 141, 111782. [Google Scholar] [CrossRef]
- Pereno, V.; Aron, M.; Vince, O.; Mannaris, C.; Seth, A.; de Saint Victor, M.; Lajoinie, G.; Versluis, M.; Coussios, C.; Carugo, D. Layered acoustofluidic resonators for the simultaneous optical and acoustic characterisation of cavitation dynamics, microstreaming, and biological effects. Biomicrofluidics 2018, 12, 034109. [Google Scholar] [CrossRef]
- Chemat, F.; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef]
- Tiwari, B.K. Ultrasound: A clean, green extraction technology. TrAC Trends Analyt. Chem. 2015, 71, 100–109. [Google Scholar] [CrossRef]
- Pan, X.; Kadla, J.F.; Ehara, K.; Gilkes, N.; Saddler, J.N. Organosolv ethanol lignin from hybrid poplar as a radical scavenger: Relationship between lignin structure, extraction conditions, and antioxidant activity. J. Agric. Food Chem. 2006, 54, 5806–5813. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Loarca-Piña, G.; Mendoza, S.; Ramos-Gómez, M.; Reynoso, R. Antioxidant, antimutagenic, and antidiabetic activities of edible leaves from Cnidoscolus chayamansa Mc. Vaugh. J. Food Sci. 2010, 75, H68–H72. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- González-Vega, R.I.; Cárdenas-López, J.L.; López-Elías, J.A.; Ruiz-Cruz, S.; Reyes-Díaz, A.; Perez-Perez, L.M.; Cinco-Moroyoqui, F.J.; Robles-Zepeda, R.E.; Borboa-Flores, J.; Del-Toro-Sánchez, C.L. Optimization of growing conditions for pigments production from microalga Navicula incerta using response surface methodology and its antioxidant capacity. Saudi J. Biol. Sci. 2021, 28, 1401–1416. [Google Scholar] [CrossRef]
- Shinde, U.; Phadke, A.; Nair, A.; Mungantiwar, A.; Dikshit, V.; Saraf, M. Studies on the anti-inflammatory and analgesic activity of Cedrus deodara (Roxb.) Loud. wood oil. J. Ethnopharmacol. 1999, 65, 21–27. [Google Scholar] [CrossRef]
- Barapatre, A.; Aadil, K.R.; Jha, H. Synergistic antibacterial and antibiofilm activity of silver nanoparticles biosynthesized by lignin-degrading fungus. Bioresour. Bioprocess. 2016, 3, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Hernandez, J.; Goycoolea, F.M.; Quintero, J.; Acosta, A.; Castañeda, M.; Dominguez, Z.; Robles, R.; Vazquez-Moreno, L.; Velazquez, E.F.; Astiazaran, H. Sonoran propolis: Chemical composition and antiproliferative activity on cancer cell lines. Planta Med. 2007, 73, 1469–1474. [Google Scholar] [CrossRef]
- Baoyuan, L.; Mahdi, M.A.-O. Catalysis in Biomass Conversion. In Advances in Inorganic Chemistry; Ford, P.C., van Eldik, R., Eds.; Academic Press; Elsevier: Amsterdam, The Netherlands, 2021; Volume 77, pp. 2–381. [Google Scholar]
- Cheng, X.-C.; Guo, X.-R.; Qin, Z.; Wang, X.-D.; Liu, H.-M.; Liu, Y.-L. Structural features and antioxidant activities of Chinese quince (Chaenomeles sinensis) fruits lignin during auto-catalyzed ethanol organosolv pretreatment. Int. J. Biol. Macromol. 2020, 164, 4348–4358. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Perez-Perez, L.M.; Armenta-Villegas, L.; Santacruz-Ortega, H.; Gutiérrez-Lomelí, M.; Aguilar, J.A.; Reynoso-Marin, F.J.; Robles-García, M.A.; Robles-Zepeda, R.E.; Ruiz-Cruz, S.; Del-Toro-Sánchez, C.L. Characterization of Anemopsis californica essential oil–β-cyclodextrin inclusion complex as antioxidant prolonged-release system. Chem. Pap. 2017, 71, 1331–1342. [Google Scholar] [CrossRef]
- Sharma, O.P.; Bhat, T.K. DPPH antioxidant assay revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Alboofetileh, M.; Rezaei, M.; Tabarsa, M.; Rittà, M.; Donalisio, M.; Mariatti, F.; You, S.; Lembo, D.; Cravotto, G. Effect of different non-conventional extraction methods on the antibacterial and antiviral activity of fucoidans extracted from Nizamuddinia zanardinii. Int. J. Biol. Macromol. 2019, 124, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, M.; Khan, M.R.; Saeed, N. Assessment of phytochemicals, antioxidant, anti-lipid peroxidation and anti-hemolytic activity of extract and various fractions of Maytenus royleanus leaves. BMC Complement. Altern. Med. 2013, 13, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suffness, M.P.; Pezzuto, J. Assays related to cancer drug discovery. In Methods in Plant Biochemistry: Assays for Bioactivity, Hostettman, K., Ed.; Academic Press: Cambridge, MA, USA, 1990; pp. 71–133. [Google Scholar]
- Moo-Puc, R.; Robledo, D.; Freile-Pelegrín, Y. In vitro cytotoxic and antiproliferative activities of marine macroalgae from Yucatán, Mexico. Cienc. Mar. 2009, 35, 345–358. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.K.; Dhepe, P.L. Isolation of lignin by organosolv process from different varieties of rice husk: Understanding their physical and chemical properties. Bioresour. Technol. 2016, 221, 310–317. [Google Scholar] [CrossRef]
- Prasetyo, I.; Permatasari, P.R.; Laksmana, W.T.; Rochmadi, R.; Oh, W.-C.; Ariyanto, T. Lignin refinery using organosolv process for nanoporous carbon synthesis. Molecules 2020, 25, 3428. [Google Scholar] [CrossRef]
- de Sousa Nascimento, L.; da Mata Vieira, F.I.D.; Horácio, V.; Marques, F.P.; Rosa, M.F.; Souza, S.A.; de Freitas, R.M.; Uchoa, D.E.A.; Mazzeto, S.E.; Lomonaco, D. Tailored organosolv banana peels lignins: Improved thermal, antioxidant and antimicrobial performances by controlling process parameters. Int. J. Biol. Macromol. 2021, 181, 241–252. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, H.; Guo, Z.; Xu, F. High purity and low molecular weight lignin nano-particles extracted from acid-assisted MIBK pretreatment. Polymers 2020, 12, 378. [Google Scholar] [CrossRef] [Green Version]
- Gosselink, R.J.; van Dam, J.E.; de Jong, E.; Scott, E.L.; Sanders, J.P.; Li, J.; Gellerstedt, G. Fractionation, analysis, and PCA modeling of properties of four technical lignins for prediction of their application potential in binders. De Gruyter 2010, 64, 193–200. [Google Scholar] [CrossRef]









| Pattern Run | Time (min) | Amplitude |
|---|---|---|
| 1 −− | 0 | 0 |
| 2 −− | 0 | 0 |
| 3 a0 | 0 | 20 |
| 4 a0 | 0 | 20 |
| 5 −+ | 0 | 20 |
| 6 −+ | 0 | 20 |
| 7 0a | 5 | 0 |
| 8 0a | 5 | 0 |
| 9 OO | 5 | 10 |
| 10 OO | 5 | 10 |
| 11 OO | 5 | 10 |
| 12 OO | 5 | 10 |
| 13 0A | 5 | 20 |
| 14 0A | 5 | 20 |
| 15 +− | 10 | 0 |
| 16 +− | 10 | 0 |
| 17 A0 | 10 | 10 |
| 18 A0 | 10 | 10 |
| 19 ++ | 10 | 20 |
| 20 ++ | 10 | 20 |
| Extraction | Initial Weight (g) | Final Weight (g) | Sample Yield (g/g) | Yield (%) |
|---|---|---|---|---|
| 1 | 30 | 0.6155 | 0.02 | 4.39 |
| 2 | 30 | 0.7493 | 0.024 | 5.35 |
| 3 | 30 | 0.8448 | 0.028 | 6.03 |
| 4 | 30 | 0.8975 | 0.029 | 6.41 |
| 5 | 30 | 1.0156 | 0.033 | 7.25 |
| 6 | 30 | 1.0427 | 0.034 | 7.44 |
| 7 | 30 | 1.2313 | 0.037 | 8.79 |
| 8 | 30 | 1.0645 | 0.035 | 7.46 |
| 9 | 30 | 1.1309 | 0.037 | 8.07 |
| 10 | 30 | 1.159 | 0.038 | 8.27 |
| 11 | 30 | 1.2762 | 0.042 | 9.11 |
| 12 | 30 | 1.2743 | 0.042 | 9.1 |
| 13 | 30 | 1.1055 | 0.036 | 7.89 |
| Average | 30 | 1.0313 ± 0.203 | 0.0334 ± 0.006 | 7.35 ± 1.45 |
| min | Amplitude (%) | DPPH | ABTS | FRAP | Phenols | Stand. Dev. | Stand. Dev. | Stand. Dev. | Stand. Dev. |
|---|---|---|---|---|---|---|---|---|---|
| μmol TE/g | μmol TE/g | μmol TE/g | mg GAE/g | DPPH | ABTS | FRAP | Phenols | ||
| 0 | 0 | 208.23 | 236.48 | 1023.96 | 395.98 | 29.37 | 53.60 | 68.84 | 58.53 |
| 0 | 0 | 205.52 | 236.48 | 1023.96 | 395.98 | 20.58 | 53.60 | 68.84 | 58.53 |
| 0 | 20 | 208.23 | 236.48 | 1023.96 | 395.98 | 29.37 | 53.60 | 68.84 | 58.53 |
| 0 | 20 | 205.52 | 236.48 | 1023.96 | 395.98 | 20.58 | 53.60 | 68.84 | 58.53 |
| 0 | 20 | 208.23 | 236.48 | 1023.96 | 395.98 | 29.37 | 53.60 | 68.84 | 58.53 |
| 0 | 20 | 205.52 | 236.48 | 1023.96 | 395.98 | 20.58 | 53.60 | 68.84 | 58.53 |
| 5 | 0 | 208.23 | 236.48 | 1023.96 | 395.98 | 29.37 | 53.60 | 68.84 | 58.53 |
| 5 | 0 | 205.52 | 236.48 | 1023.96 | 395.98 | 20.58 | 53.60 | 68.84 | 58.53 |
| 5 | 10 | 211.72 | 243.95 | 1084.66 | 428.28 | 61.45 | 56.88 | 97.33 | 122.77 |
| 5 | 10 | 189.45 | 243.95 | 1084.66 | 428.28 | 14.90 | 56.88 | 97.33 | 122.77 |
| 5 | 10 | 211.72 | 243.95 | 1084.66 | 428.28 | 61.45 | 56.88 | 97.33 | 122.77 |
| 5 | 10 | 189.45 | 243.95 | 1084.66 | 428.28 | 14.90 | 56.88 | 97.33 | 122.77 |
| 5 | 20 | 217.33 | 257.29 | 1624.60 | 467.84 | 31.94 | 20.18 | 289.17 | 54.10 |
| 5 | 20 | 191.00 | 257.29 | 1624.60 | 467.84 | 23.64 | 20.18 | 289.17 | 54.10 |
| 10 | 0 | 208.23 | 236.48 | 1023.96 | 395.98 | 29.37 | 53.60 | 68.84 | 58.53 |
| 10 | 0 | 205.52 | 236.48 | 1023.96 | 395.98 | 20.58 | 53.60 | 68.84 | 58.53 |
| 10 | 10 | 266.32 | 273.25 | 1386.58 | 483.37 | 121.50 | 46.80 | 294.03 | 100.84 |
| 10 | 10 | 169.51 | 273.25 | 1386.58 | 483.37 | 41.15 | 46.80 | 294.03 | 100.84 |
| 10 | 20 | 227.40 | 257.09 | 1694.88 | 420.52 | 37.39 | 53.87 | 193.39 | 26.22 |
| 10 | 20 | 210.75 | 257.09 | 1694.88 | 420.52 | 64.82 | 53.87 | 193.39 | 26.22 |
| Optimization Results | |||||||
|---|---|---|---|---|---|---|---|
| DPPH | ABTS | FRAP | Total phenols | ||||
| Time (min) | Amplitude (%) | Time (min) | Amplitude (%) | Time (min) | Amplitude (%) | Time (min) | Amplitude (%) |
| 10 | 20 | 10 | 18 | 10 | 20 | 10 | 14 |
| Cellular Line | LCS | L1 | L2 | L3 |
|---|---|---|---|---|
| HeLa | 252.07 ± 28.56 | 284.90 ± 23.84 | 253.92 ± 3.98 | 253.14 ± 10.05 |
| PC-3 | 126.52 ± 4.83 | 171.85 ± 18.91 | 196.06 ± 12.47 | 209.28 ± 12.66 |
| L-929 | ND | ND | ND | ND |
| Wave Number (cm−1) | Description |
|---|---|
| 3276 | O–H tension vibration of alcohol and phenol groups |
| 1655 | C=O tension vibration |
| 1605 | C=O stretching of the aromatic ring |
| 1513 | Syringile ring vibration and C–O tension vibration |
| 1470 | Guayacil ring vibration and C–O tension vibration |
| 1295 | C–H stretch of guayacil units (G) |
| 1167 | Units p-hydroxyphenyl (H), guaiacil G and syringyl (S) |
| 1122–1056 | C–H bond strain in the aromatic ring |
| 947–793 | C–H vibration deformation in associated aromatic rings Bending C–H of the syringil units. p-substituted phenolics |
| Sample | tR (min) | Mw (Da) |
|---|---|---|
| LCS | 11.246 | 2401.19 |
| L1 | 11.311 | 2334.20 |
| L2 | 11.354 | 2130.17 |
| L3 | 11.282 | 2307.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reyna-Reyna, L.Y.; Montaño-Leyva, B.; Valencia, D.; Cinco-Moroyoqui, F.J.; González-Vega, R.I.; Bernal-Mercado, A.T.; Ballesteros-Monrreal, M.G.; Mendez-Encinas, M.A.; Del-Toro-Sánchez, C.L. Antioxidant, Antibacterial, Anti-Inflammatory, and Antiproliferative Activity of Sorghum Lignin (Sorghum bicolor) Treated with Ultrasonic Pulses. Metabolites 2023, 13, 394. https://doi.org/10.3390/metabo13030394
Reyna-Reyna LY, Montaño-Leyva B, Valencia D, Cinco-Moroyoqui FJ, González-Vega RI, Bernal-Mercado AT, Ballesteros-Monrreal MG, Mendez-Encinas MA, Del-Toro-Sánchez CL. Antioxidant, Antibacterial, Anti-Inflammatory, and Antiproliferative Activity of Sorghum Lignin (Sorghum bicolor) Treated with Ultrasonic Pulses. Metabolites. 2023; 13(3):394. https://doi.org/10.3390/metabo13030394
Chicago/Turabian StyleReyna-Reyna, Linda Yareth, Beatriz Montaño-Leyva, Dora Valencia, Francisco Javier Cinco-Moroyoqui, Ricardo Iván González-Vega, Ariadna Thalía Bernal-Mercado, Manuel G. Ballesteros-Monrreal, Mayra A. Mendez-Encinas, and Carmen Lizette Del-Toro-Sánchez. 2023. "Antioxidant, Antibacterial, Anti-Inflammatory, and Antiproliferative Activity of Sorghum Lignin (Sorghum bicolor) Treated with Ultrasonic Pulses" Metabolites 13, no. 3: 394. https://doi.org/10.3390/metabo13030394
APA StyleReyna-Reyna, L. Y., Montaño-Leyva, B., Valencia, D., Cinco-Moroyoqui, F. J., González-Vega, R. I., Bernal-Mercado, A. T., Ballesteros-Monrreal, M. G., Mendez-Encinas, M. A., & Del-Toro-Sánchez, C. L. (2023). Antioxidant, Antibacterial, Anti-Inflammatory, and Antiproliferative Activity of Sorghum Lignin (Sorghum bicolor) Treated with Ultrasonic Pulses. Metabolites, 13(3), 394. https://doi.org/10.3390/metabo13030394