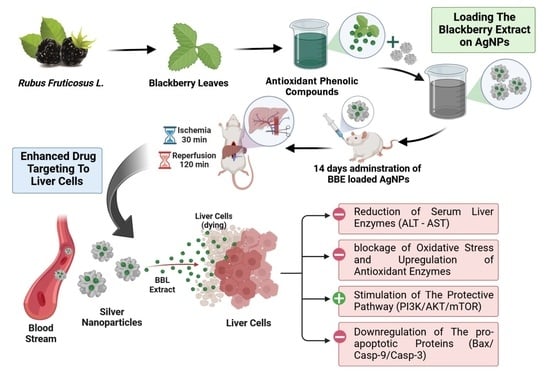
Blackberry-Loaded AgNPs Attenuate Hepatic Ischemia/Reperfusion Injury via PI3K/Akt/mTOR Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Kits and Antibodies
2.3. Preparation of Blackberry Extract
2.4. LC–HR–ESI–MS Metabolic Profiling of Blackberry Leaves Extract
2.5. Solid State Synthesis of AgNPs
2.6. Preparation of Blackberry Silver Nitrate Nanoparticles (BBE-AgNPs)
2.7. Characterization of the Prepared Metal Nanoparticles
2.8. Entrapment Efficiency
2.9. Hepatic Ischemia/Reperfusion Surgery Model
2.10. Experimental Design
2.11. Preparation of Tissue Homogenate
2.12. Biochemical Analysis
2.12.1. Determination of Liver Function Parameters
2.12.2. Assessment of Oxidant and Antioxidant Markers
2.13. Histopathological Study
2.14. Western Blotting
2.15. Immunohistochemistry
2.16. In Silico Study
2.16.1. Ischemia Potential Protein Targets Determination
2.16.2. Molecular Dynamic Simulation and Binding Free Energy Calculation
2.17. Statistical Analysis
3. Results
3.1. LC–HR–ESI–MS Chemical Profiling of BBE
3.2. Characterization of the Prepared BBE-AgNPs Nanoparticles
3.3. Entrapment Efficiency of the Prepared BBE-AgNPs Nanoparticles
3.4. Serum ALT and AST Activities
3.5. Hepatic Lipid Peroxidation and Antioxidant Markers
3.6. Histopathological Analysis
3.7. Effect of BBE-AgNPs on Cleaved Caspase-3, p-PI3k, p-Akt, p-mTOR Protein Expression
3.8. Effect of BBE-AgNPs on Bax and Cleaved Caspase-9 Protein Expression
3.9. In Silico Study
Identifying the Likely Molecular Target of BBE’s Dereplicated Natural Products
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lentsch, A.B.; Kato, A.; Yoshidome, H.; McMasters, K.M.; Edwards, M.J. Inflammatory mechanisms and therapeutic strategies for warm hepatic ischemia/reperfusion injury. Hepatology 2000, 32, 169–173. [Google Scholar] [CrossRef]
- Fondevila, C.; Busuttil, R.W.; Kupiec-Weglinski, J.W. Hepatic ischemia/reperfusion injury—A fresh look. Exp. Mol. Pathol. 2003, 74, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Clavien, P.A.; Rüdiger, H.A.; Selzner, M. Mechanism of hepatocyte death after ischemia: Apoptosis versus necrosis. Hepatology 2001, 33, 1555–1556. [Google Scholar] [CrossRef]
- Datta, G.; Fuller, B.J.; Davidson, B.R. Molecular mechanisms of liver ischemia reperfusion injury: Insights from transgenic knockout models. World J. Gastroenterol. WJG 2013, 19, 1683. [Google Scholar] [CrossRef]
- Zhai, Y.; Petrowsky, H.; Hong, J.C.; Busuttil, R.W.; Kupiec-Weglinski, J.W. Ischaemia–reperfusion injury in liver transplantation—From bench to bedside. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 79–89. [Google Scholar] [CrossRef]
- Cursio, R.; Colosetti, P.; Gugenheim, J. Autophagy and liver ischemia-reperfusion injury. BioMed Res. Int. 2015, 2015, 417590. [Google Scholar] [CrossRef] [PubMed]
- Lemasters, J.J.; Thurman, R.G. Reperfusion injury after liver preservation for transplantation. Annu. Rev. Pharmacol. Toxicol. 1997, 37, 327–338. [Google Scholar] [CrossRef]
- Thompson, C.B. Apoptosis in the pathogenesis and treatment of disease. Science 1995, 267, 1456–1462. [Google Scholar] [CrossRef]
- Noda, T.; Iwakiri, R.; Fujimoto, K.; Matsuo, S.; Aw, T.Y. Programmed cell death induced by ischemia-reperfusion in rat intestinal mucosa. Am. J. Physiol.-Gastrointest. Liver Physiol. 1998, 274, G270–G276. [Google Scholar] [CrossRef]
- Kalimuthu, S.; Se-Kwon, K. Cell survival and apoptosis signaling as therapeutic target for cancer: Marine bioactive compounds. Int. J. Mol. Sci. 2013, 14, 2334–2354. [Google Scholar] [CrossRef] [Green Version]
- Weinberg, S.E.; Chandel, N.S. Targeting mitochondria metabolism for cancer therapy. Nat. Chem. Biol. 2015, 11, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rezq, S.; Hassan, R.; Mahmoud, M.F. Rimonabant ameliorates hepatic ischemia/reperfusion injury in rats: Involvement of autophagy via modulating ERK-and PI3K/AKT-mTOR pathways. Int. Immunopharmacol. 2021, 100, 108140. [Google Scholar] [CrossRef]
- Jia, S.; Zhang, H.; Li, L.; Wang, F.; Zhang, B. Shogaol potentiates sevoflurane mediated neuroprotection against ischemia/reperfusion-induced brain injury via regulating apoptotic proteins and PI3K/Akt/mTOR/s6K signalling and HIF-1α/HO-1 expression. Saudi J. Biol. Sci. 2021, 28, 5002–5010. [Google Scholar] [CrossRef]
- Wei, Q.; Zhao, J.; Zhou, X.; Yu, L.; Liu, Z.; Chang, Y. Propofol can suppress renal ischemia-reperfusion injury through the activation of PI3K/AKT/mTOR signal pathway. Gene 2019, 708, 14–20. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, J.; Zhang, J.; Sun, K.; Li, Q.; Kuang, B.; Wang, M.Z.; Hou, S.; Gong, N. Methyl eugenol attenuates liver ischemia reperfusion injury via activating PI3K/Akt signaling. Int. Immunopharmacol. 2021, 99, 108023. [Google Scholar] [CrossRef]
- Liu, C.; Chen, K.; Wang, H.; Zhang, Y.; Duan, X.; Xue, Y.; He, H.; Huang, Y.; Chen, Z.; Ren, H. Gastrin attenuates renal ischemia/reperfusion injury by a PI3K/Akt/Bad-mediated anti-apoptosis signaling. Front. Pharmacol. 2020, 11, 540479. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Hsiao, J.-K.; Lu, Y.-Z.; Lee, T.-C.; Linda, C. Anti-apoptotic PI3K/Akt signaling by sodium/glucose transporter 1 reduces epithelial barrier damage and bacterial translocation in intestinal ischemia. Lab. Investig. 2011, 91, 294–309. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.J.; Joe, Y.; Kong, J.S.; Jeong, S.-O.; Cho, G.J.; Ryter, S.W.; Chung, H.T. Carbon monoxide protects against hepatic ischemia/reperfusion injury via ROS-dependent Akt signaling and inhibition of glycogen synthase kinase 3β. Oxidative Med. Cell. Longev. 2013, 2013, 306421. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Zhang, L.; Manaenko, A.; Ye, Z.; Liu, W.; Sun, X. Helium preconditioning protects mouse liver against ischemia and reperfusion injury through the PI3K/Akt pathway. J. Hepatol. 2014, 61, 1048–1055. [Google Scholar] [CrossRef]
- Liu, H.; Li, J.; Jiang, C.; Yuan, T.; Ma, H. Cellular communication network factor 1 (CCN1) knockdown exerts a protective effect for hepatic ischemia/reperfusion injury by deactivating the MEK/ERK pathway. Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101737. [Google Scholar] [CrossRef]
- Wang, W.; Wu, L.; Li, J.; Ji, J.; Chen, K.; Yu, Q.; Li, S.; Feng, J.; Liu, T.; Zhang, J. Alleviation of hepatic ischemia reperfusion injury by oleanolic acid pretreating via reducing HMGB1 release and inhibiting apoptosis and autophagy. Mediat. Inflamm. 2019, 2019, 3240713. [Google Scholar] [CrossRef]
- Li, Y.; Tong, L.; Zhang, J.; Zhang, Y.; Zhang, F. Galangin alleviates liver ischemia-reperfusion injury in a rat model by mediating the PI3K/AKT pathway. Cell Physiol. Biochem. 2018, 51, 1354–1363. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Rodriguez, E.O.; Yousef, G.G.; Garcia-Saucedo, P.A.; Lopez-Medina, J.; Paredes-Lopez, O.; Lila, M.A. Characterization of anthocyanins and proanthocyanidins in wild and domesticated Mexican blackberries (Rubus spp.). J. Agric. Food Chem. 2010, 58, 7458–7464. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.O.; Lee, C.-W.; So, Y.; Jin, C.-H.; Yook, H.-S.; Byun, M.-W.; Jeong, Y.-W.; Park, J.C.; Jeong, I.-Y. Protective effects of radiation-induced blackberry mutant extract on carbon tetrachloride (CCl 4)-induced liver injury in sprague-dawley rats. J. Korean Soc. Food Sci. Nutr. 2014, 43, 807–813. [Google Scholar] [CrossRef]
- Park, S.; Cho, S.M.; Jin, B.R.; Yang, H.J.; Yi, Q.J. Mixture of blackberry leaf and fruit extracts alleviates non-alcoholic steatosis, enhances intestinal integrity, and increases Lactobacillus and Akkermansia in rats. Exp. Biol. Med. 2019, 244, 1629–1641. [Google Scholar] [CrossRef] [Green Version]
- Won, R.H.; Ok, C.B.; Yeong, B.J.; Hyun, J.C.; Seong, C.D.; Yun, J.I. Hepatorotective Effect of Blackberry Extract on Carbon Tetrachloride-induced Liver Injury in Rats. Kbb Scholar. 2011. Available online: https://scholar.kyobobook.co.kr/article/detail/4010024649493 (accessed on 14 February 2023).
- Bowen-Forbes, C.S.; Zhang, Y.; Nair, M.G. Anthocyanin content, antioxidant, anti-inflammatory and anticancer properties of blackberry and raspberry fruits. J. Food Compos. Anal. 2010, 23, 554–560. [Google Scholar] [CrossRef]
- Cuevas-Rodriguez, E.O.; Dia, V.P.; Yousef, G.G.; Garcia-Saucedo, P.A.; López-Medina, J.; Paredes-Lopez, O.; Gonzalez de Mejia, E.; Lila, M.A. Inhibition of pro-inflammatory responses and antioxidant capacity of Mexican blackberry (Rubus spp.) extracts. J. Agric. Food Chem. 2010, 58, 9542–9548. [Google Scholar] [CrossRef]
- Srivastava, A.; Greenspan, P.; Hartle, D.K.; Hargrove, J.L.; Amarowicz, R.; Pegg, R.B. Antioxidant and anti-inflammatory activities of polyphenolics from southeastern US range blackberry cultivars. J. Agric. Food Chem. 2010, 58, 6102–6109. [Google Scholar] [CrossRef]
- Hassimotto, N.M.; Lajolo, F.M. Antioxidant status in rats after long-term intake of anthocyanins and ellagitannins from blackberries. J. Sci. Food Agric. 2011, 91, 523–531. [Google Scholar] [CrossRef]
- Pergola, C.; Rossi, A.; Dugo, P.; Cuzzocrea, S.; Sautebin, L. Inhibition of nitric oxide biosynthesis by anthocyanin fraction of blackberry extract. Nitric Oxide 2006, 15, 30–39. [Google Scholar] [CrossRef] [Green Version]
- Shin, W.-H.; Park, S.-J.; Kim, E.-J. Protective effect of anthocyanins in middle cerebral artery occlusion and reperfusion model of cerebral ischemia in rats. Life Sci. 2006, 79, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Tsoy, I.; Park, J.M.; Chung, J.I.; Shin, S.C.; Chang, K.C. Anthocyanins from soybean seed coat inhibit the expression of TNF-α-induced genes associated with ischemia/reperfusion in endothelial cell by NF-κB-dependent pathway and reduce rat myocardial damages incurred by ischemia and reperfusion in vivo. FEBS Lett. 2006, 580, 1391–1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, M.; Feng, R.; Wang, S.Y.; Bowman, L.; Lu, Y.; Qian, Y.; Castranova, V.; Jiang, B.-H.; Shi, X. Cyanidin-3-glucoside, a natural product derived from blackberry, exhibits chemopreventive and chemotherapeutic activity. J. Biol. Chem. 2006, 281, 17359–17368. [Google Scholar] [CrossRef] [Green Version]
- Trinei, M.; Carpi, A.; Storto, M.; Fornari, M.; Marinelli, A.; Minardi, S.; Riboni, M.; Casciaro, F.; DiLisa, F.; Petroni, K. Dietary intake of cyanidin-3-glucoside induces a long-lasting cardioprotection from ischemia/reperfusion injury by altering the microbiota. J. Nutr. Biochem. 2022, 101, 108921. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T.; Horio, F.; Kitoh, J.; Osawa, T. Protective effects of dietary cyanidin 3-O-β-d-glucoside on liver ischemia–reperfusion injury in rats. Arch. Biochem. Biophys. 1999, 368, 361–366. [Google Scholar] [CrossRef]
- Hussein, J.; El-Naggar, M.E.; Fouda, M.M.; Morsy, O.M.; Ajarem, J.S.; Almalki, A.M.; Allam, A.A.; Mekawi, E.M. The efficiency of blackberry loaded AgNPs, AuNPs and Ag@ AuNPs mediated pectin in the treatment of cisplatin-induced cardiotoxicity in experimental rats. Int. J. Biol. Macromol. 2020, 159, 1084–1093. [Google Scholar] [CrossRef]
- Hussein, J.; El-Naggar, M.E.; Latif, Y.A.; Medhat, D.; El Bana, M.; Refaat, E.; Morsy, S. Solvent-free and one-pot synthesis of silver and zinc oxide nanoparticles: Activity toward cell membrane component and insulin signaling pathway in experimental diabetes. Colloids Surf. B Biointerfaces 2018, 170, 76–84. [Google Scholar] [CrossRef]
- Abdelgawad, A.M.; El-Naggar, M.E.; Eisa, W.H.; Rojas, O.J. Clean and high-throughput production of silver nanoparticles mediated by soy protein via solid state synthesis. J. Clean. Prod. 2017, 144, 501–510. [Google Scholar] [CrossRef]
- Hebeish, A.; Shaheen, T.I.; El-Naggar, M.E. Solid state synthesis of starch-capped silver nanoparticles. Int. J. Biol. Macromol. 2016, 87, 70–76. [Google Scholar] [CrossRef]
- Elisia, I.; Hu, C.; Popovich, D.G.; Kitts, D.D. Antioxidant assessment of an anthocyanin-enriched blackberry extract. Food Chem. 2007, 101, 1052–1058. [Google Scholar] [CrossRef]
- Abdelmohsen, U.R.; Cheng, C.; Viegelmann, C.; Zhang, T.; Grkovic, T.; Ahmed, S.; Quinn, R.J.; Hentschel, U.; Edrada-Ebel, R. Dereplication strategies for targeted isolation of new antitrypanosomal actinosporins A and B from a marine sponge associated-Actinokineospora sp. EG49. Mar. Drugs 2014, 12, 1220–1244. [Google Scholar] [CrossRef] [Green Version]
- Abdelhafez, O.H.; Othman, E.M.; Fahim, J.R.; Desoukey, S.Y.; Pimentel-Elardo, S.M.; Nodwell, J.R.; Schirmeister, T.; Tawfike, A.; Abdelmohsen, U.R. Metabolomics analysis and biological investigation of three Malvaceae plants. Phytochem. Anal. 2020, 31, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Natsuki, J.; Natsuki, T.; Hashimoto, Y. A review of silver nanoparticles: Synthesis methods, properties and applications. Int. J. Mater. Sci. Appl. 2015, 4, 325–332. [Google Scholar] [CrossRef] [Green Version]
- Cho, B.O.; Ryu, H.W.; Jin, C.H.; Choi, D.S.; Kang, S.Y.; Kim, D.S.; Byun, M.-W.; Jeong, I.Y. Blackberry extract attenuates oxidative stress through up-regulation of Nrf2-dependent antioxidant enzymes in carbon tetrachloride-treated rats. J. Agric. Food Chem. 2011, 59, 11442–11448. [Google Scholar] [CrossRef]
- Younis, N.N.; Shaheen, M.A.; Mahmoud, M.F. Silymarin preconditioning protected insulin resistant rats from liver ischemia-reperfusion injury: Role of endogenous H2S. J. Surg. Res. 2016, 204, 398–409. [Google Scholar] [CrossRef]
- Rao, P.R.; Viswanath, R.K. Cardioprotective activity of silymarin in ischemia-reperfusion-induced myocardial infarction in albino rats. Exp. Clin. Cardiol. 2007, 12, 179. [Google Scholar] [PubMed]
- Shaker, E.; Mahmoud, H.; Mnaa, S. Silymarin, the antioxidant component and Silybum marianum extracts prevent liver damage. Food Chem. Toxicol. 2010, 48, 803–806. [Google Scholar] [CrossRef]
- Reitman, S.; Frankel, S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957, 28, 56–63. [Google Scholar] [CrossRef]
- Guo, Y.; Yang, T.; Lu, J.; Li, S.; Wan, L.; Long, D.; Li, Q.; Feng, L.; Li, Y. Rb1 postconditioning attenuates liver warm ischemia–reperfusion injury through ROS-NO-HIF pathway. Life Sci. 2011, 88, 598–605. [Google Scholar] [CrossRef]
- Youssef, N.S.; Elzatony, A.S.; Baky, N.A.A. Diacerein attenuate LPS-induced acute lung injury via inhibiting ER stress and apoptosis: Impact on the crosstalk between SphK1/S1P, TLR4/NFκB/STAT3, and NLRP3/IL-1β signaling pathways. Life Sci. 2022, 308, 120915. [Google Scholar] [CrossRef]
- Waz, S.; Heeba, G.H.; Hassanin, S.O.; Abdel-Latif, R.G. Nephroprotective effect of exogenous hydrogen sulfide donor against cyclophosphamide-induced toxicity is mediated by Nrf2/HO-1/NF-κB signaling pathway. Life Sci. 2021, 264, 118630. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-C.; Chu, P.-Y.; Chen, C.-M.; Lin, J.-H. idTarget: A web server for identifying protein targets of small chemical molecules with robust scoring functions and a divide-and-conquer docking approach. Nucleic Acids Res. 2012, 40, W393–W399. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, M.S.; Abdelkader, K.; Gomaa, H.A.; Batubara, A.S.; Gamal, M.; Sayed, A.M. Mechanistic study of the antibacterial potential of the prenylated flavonoid auriculasin against Escherichia coli. Arch. Pharm. 2022, 355, e2200360. [Google Scholar] [CrossRef] [PubMed]
- Martin-Rivilla, H.; Garcia-Villaraco, A.; Ramos-Solano, B.; Gutierrez-Manero, F.; Lucas, J. Improving flavonoid metabolism in blackberry leaves and plant fitness by using the bioeffector Pseudomonas fluorescens N 21.4 and its metabolic elicitors: A biotechnological approach for a more sustainable crop. J. Agric. Food Chem. 2020, 68, 6170–6180. [Google Scholar] [CrossRef] [PubMed]
- Natsuki, J.; Abe, T. Synthesis of pure colloidal silver nanoparticles with high electroconductivity for printed electronic circuits: The effect of amines on their formation in aqueous media. J. Colloid Interface Sci. 2011, 359, 19–23. [Google Scholar] [CrossRef]
- Hou, S.; Xu, T.; Xu, J.; Qu, L.; Xu, Y.; Chen, L.; Liu, J. Structural basis for functional selectivity and ligand recognition revealed by crystal structures of human secreted phospholipase A2 group IIE. Sci. Rep. 2017, 7, 10815. [Google Scholar] [CrossRef]
- Ogata, K.; Jin, M.B.; Taniguchi, M.; Suzuki, T.; Shimamura, T.; Kitagawa, N.; Magata, S.; Fukai, M.; Ishikawa, H.; Ono, T. Attenuation of ischemia and reperfusion injury of canine livers by inhibition of type II phospholipase A2 with Ly3297221. Transplantation 2001, 71, 1040–1046. [Google Scholar] [CrossRef]
- Adibhatla, R.M.; Hatcher, J. Phospholipase A2, reactive oxygen species, and lipid peroxidation in cerebral ischemia. Free Radic. Biol. Med. 2006, 40, 376–387. [Google Scholar] [CrossRef]
- Inoue, S.; Sugitani, A.; Yamamoto, H.; Kitada, H.; Motoyama, K.; Okabe, Y.; Ohta, M.; Yoshida, J.-i.; Nishiyama, K.-i.; Tanaka, M. Effect of synthetic protease inhibitor gabexate mesilate on the attenuation of ischemia/reperfusion injury in canine kidney autotransplantation. Surgery 2005, 137, 216–224. [Google Scholar] [CrossRef]
- Dreiseitel, A.; Korte, G.; Schreier, P.; Oehme, A.; Locher, S.; Hajak, G.; Sand, P.G. sPhospholipase A2 is inhibited by anthocyanidins. J. Neural Transm. 2009, 116, 1071–1077. [Google Scholar] [CrossRef]
- Hassimotto, N.M.A.; Moreira, V.; Nascimento, N.G.d.; Souto, P.C.M.d.C.; Teixeira, C.; Lajolo, F.M. Inhibition of carrageenan-induced acute inflammation in mice by oral administration of anthocyanin mixture from wild mulberry and cyanidin-3-glucoside. BioMed Res. Int. 2013, 2013, 146716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitjavila, M.; Moreno, J. The effects of polyphenols on oxidative stress and the arachidonic acid cascade. Implications for the prevention/treatment of high prevalence diseases. Biochem. Pharmacol. 2012, 84, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Simonyi, A.; He, Y.; Sheng, W.; Sun, A.Y.; Wood, W.G.; Weisman, G.A.; Sun, G.Y. Targeting NADPH oxidase and phospholipases A2 in Alzheimer’s disease. Mol. Neurobiol. 2010, 41, 73–86. [Google Scholar] [CrossRef] [Green Version]
- De Rougemont, O.; Lehmann, K.; Clavien, P.A. Preconditioning, organ preservation, and postconditioning to prevent ischemia-reperfusion injury to the liver. Liver Transplant. 2009, 15, 1172–1182. [Google Scholar] [CrossRef]
- Zia-Ul-Haq, M.; Riaz, M.; De Feo, V.; Jaafar, H.Z.; Moga, M. Rubus fruticosus L.: Constituents, biological activities and health related uses. Molecules 2014, 19, 10998–11029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, R.; Gangrade, T.; Punasiya, R.; Ghulaxe, C. Rubus fruticosus (blackberry) use as an herbal medicine. Pharmacogn. Rev. 2014, 8, 101. [Google Scholar] [CrossRef] [Green Version]
- Paczkowska-Walendowska, M.; Gościniak, A.; Szymanowska, D.; Szwajgier, D.; Baranowska-Wójcik, E.; Szulc, P.; Dreczka, D.; Simon, M.; Cielecka-Piontek, J. Blackberry Leaves as New Functional Food? Screening Antioxidant, Anti-Inflammatory and Microbiological Activities in Correlation with Phytochemical Analysis. Antioxidants 2021, 10, 1945. [Google Scholar] [CrossRef]
- Mu, J.; Li, C.; Shi, Y.; Liu, G.; Zou, J.; Zhang, D.-Y.; Jiang, C.; Wang, X.; He, L.; Huang, P. Protective effect of platinum nano-antioxidant and nitric oxide against hepatic ischemia-reperfusion injury. Nat. Commun. 2022, 13, 1–12. [Google Scholar]
- Kalhapure, R.S.; Suleman, N.; Mocktar, C.; Seedat, N.; Govender, T. Nanoengineered drug delivery systems for enhancing antibiotic therapy. J. Pharm. Sci. 2015, 104, 872–905. [Google Scholar] [CrossRef]
- Refaat, H.; Mady, F.M.; Sarhan, H.A.; Rateb, H.S.; Alaaeldin, E. Optimization and evaluation of propolis liposomes as a promising therapeutic approach for COVID-19. Int. J. Pharm. 2021, 592, 120028. [Google Scholar] [CrossRef]
- Refaat, H.; Naguib, Y.W.; Elsayed, M.M.; Sarhan, H.A.; Alaaeldin, E. Modified spraying technique and response surface methodology for the preparation and optimization of propolis liposomes of enhanced anti-proliferative activity against human melanoma cell line A375. Pharmaceutics 2019, 11, 558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musa, A.; Elmaidomy, A.H.; Sayed, A.M.; Alzarea, S.I.; Al-Sanea, M.M.; Mostafa, E.M.; Hendawy, O.M.; Abdelgawad, M.A.; Youssif, K.A.; Refaat, H. Cytotoxic potential, metabolic profiling, and liposomes of Coscinoderma sp. crude extract supported by in silico analysis. Int. J. Nanomed. 2021, 16, 3861. [Google Scholar] [CrossRef] [PubMed]
- Owis, A.I.; El-Hawary, M.S.; El Amir, D.; Refaat, H.; Alaaeldin, E.; Aly, O.M.; Elrehany, M.A.; Kamel, M.S. Flavonoids of Salvadora persica L.(meswak) and its liposomal formulation as a potential inhibitor of SARS-CoV-2. RSC Adv. 2021, 11, 13537–13544. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, R.D.; Apostol, J.G.; de Leon, J.D.; Mariano, J.D.; Mirhan, C.M.C.; Pangan, S.S.; Reyes, A.G.M.; Zamora, E.T. Polysaccharide-mediated green synthesis of silver nanoparticles from Sargassum siliquosum JG Agardh: Assessment of toxicity and hepatoprotective activity. OpenNano 2016, 1, 16–24. [Google Scholar] [CrossRef] [Green Version]
- Eswaran, A.; Muthukrishnan, S.; Mathaiyan, M.; Pradeepkumar, S.; Mari, K.R.; Manogaran, P. Green synthesis, characterization and hepatoprotective activity of silver nanoparticles synthesized from pre-formulated Liv-Pro-08 poly-herbal formulation. Appl. Nanosci. 2023, 13, 2315–2327. [Google Scholar] [CrossRef]
- Zhang, H.; Jacob, J.A.; Jiang, Z.; Xu, S.; Sun, K.; Zhong, Z.; Varadharaju, N.; Shanmugam, A. Hepatoprotective effect of silver nanoparticles synthesized using aqueous leaf extract of Rhizophora apiculata. Int. J. Nanomed. 2019, 14, 3517. [Google Scholar] [CrossRef] [Green Version]
- Van der Zande, M.; Vandebriel, R.J.; Van Doren, E.; Kramer, E.; Herrera Rivera, Z.; Serrano-Rojero, C.S.; Gremmer, E.R.; Mast, J.; Peters, R.J.; Hollman, P.C. Distribution, elimination, and toxicity of silver nanoparticles and silver ions in rats after 28-day oral exposure. ACS Nano 2012, 6, 7427–7442. [Google Scholar] [CrossRef]
- Xu, M.; Yang, Q.; Xu, L.; Rao, Z.; Cao, D.; Gao, M.; Liu, S. Protein target identification and toxicological mechanism investigation of silver nanoparticles-induced hepatotoxicity by integrating proteomic and metallomic strategies. Part. Fibre Toxicol. 2019, 16, 46. [Google Scholar] [CrossRef] [Green Version]
- Akbari-Kordkheyli, V.; Abbaszadeh-Goudarzi, K.; Nejati-Laskokalayeh, M.; Zarpou, S.; Khonakdar-Tarsi, A. The protective effects of silymarin on ischemia-reperfusion injuries: A mechanistic review. Iran. J. Basic Med. Sci. 2019, 22, 968. [Google Scholar]
- Kamiike, W.; Fujikawa, M.; Koseki, M.; Sumimura, J.; Miyata, M.; Kawashima, Y.; Wada, H.; Tagawa, K. Different patterns of leakage of cytosolic and mitochondrial enzymes. Clin. Chim. Acta 1989, 185, 265–270. [Google Scholar] [CrossRef]
- Elias-Miro, M.; Jiménez-Castro, M.B.; Rodés, J.; Peralta, C. Current knowledge on oxidative stress in hepatic ischemia/reperfusion. Free Radic. Res. 2013, 47, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, T.A.F.; Navarro, B.C.H.; Pérez, J.A.M. Endogenous antioxidants: A review of their role in oxidative stress. In A Master Regulator of Oxidative Stress—The Transcription Factor Nrf2; IntechOpen Limited: London, UK, 2016. [Google Scholar] [CrossRef] [Green Version]
- Henkel, R.; Samanta, L.; Agarwal, A. Oxidants, Antioxidants, and Impact of the Oxidative Status in Male Reproduction; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Lu, X.-L.; Zhao, C.-H.; Yao, X.-L.; Zhang, H. Quercetin attenuates high fructose feeding-induced atherosclerosis by suppressing inflammation and apoptosis via ROS-regulated PI3K/AKT signaling pathway. Biomed. Pharmacother. 2017, 85, 658–671. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, Q.; Mo, W.; Yu, Q.; Xu, S.; Li, J.; Li, S.; Feng, J.; Wu, L.; Lu, X. The protective effects of shikonin on hepatic ischemia/reperfusion injury are mediated by the activation of the PI3K/Akt pathway. Sci. Rep. 2017, 7, 44785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seervi, M.; Rani, A.; Sharma, A.K.; Kumar, T.S. ROS mediated ER stress induces Bax-Bak dependent and independent apoptosis in response to Thioridazine. Biomed. Pharmacother. 2018, 106, 200–209. [Google Scholar] [CrossRef]
- Ben-Ari, Z.; Pappo, O.; Cheporko, Y.; Yasovich, N.; Offen, D.; Shainberg, A.; Leshem, D.; Sulkes, J.; Vidne, B.A.; Hochhauser, E. Bax ablation protects against hepatic ischemia/reperfusion injury in transgenic mice. Liver Transplant. 2007, 13, 1181–1188. [Google Scholar] [CrossRef]
- Crowley, L.C.; Waterhouse, N.J. Detecting cleaved caspase-3 in apoptotic cells by flow cytometry. Cold Spring Harb. Protoc. 2016, 2016, pdb-prot087312. [Google Scholar] [CrossRef]
- Zha, H.; Reed, J.C. Heterodimerization-independent functions of cell death regulatory proteins Bax and Bcl-2 in yeast and mammalian cells. J. Biol. Chem. 1997, 272, 31482–31488. [Google Scholar] [CrossRef] [Green Version]
- Rentsch, M.; Beham, A.; Lesalnieks, I.; Mirwald, T.; Anthuber, M. Impact of prolonged cold ischemia and reperfusion on apoptosis, activation of caspase 3, and expression of bax after liver transplantation in the rat. Transplant. Proc. 2001, 33, 850–851. [Google Scholar] [CrossRef]
- Peralta, C.; Jiménez-Castro, M.B.; Gracia-Sancho, J. Hepatic ischemia and reperfusion injury: Effects on the liver sinusoidal milieu. J. Hepatol. 2013, 59, 1094–1106. [Google Scholar] [CrossRef] [Green Version]
- Hamad, S.M.; Shnawa, B.H.; Jalil, P.J.; Ahmed, M.H. Assessment of the Therapeutic Efficacy of Silver Nanoparticles against Secondary Cystic Echinococcosis in BALB/c Mice. Surfaces 2022, 5, 91–112. [Google Scholar] [CrossRef]
- Sardari, R.R.R.; Zarchi, S.R.; Talebi, A.; Nasri, S.; Imani, S.; Khoradmehr, A.; Sheshde, S.A.R. Toxicological effects of silver nanoparticles in rats. Afr. J. Microbiol. Res. 2012, 6, 5587–5593. [Google Scholar]
- Alalaiwe, A. The clinical pharmacokinetics impact of medical nanometals on drug delivery system. Nanomed. Nanotechnol. Biol. Med. 2019, 17, 47–61. [Google Scholar] [CrossRef]
- Bowers, K.J.; Chow, D.E.; Xu, H.; Dror, R.O.; Eastwood, M.P.; Gregersen, B.A.; Klepeis, J.L.; Kolossvary, I.; Moraes, M.A.; Sacerdoti, F.D.; et al. Scalable algorithms for molecular dynamics simulations on commodity clusters. In Proceedings of the SC ‘06: Proceedings of the 2006 ACM/IEEE Conference on Supercomputing, Tampa, FL, USA, 11–17 November 2006. [Google Scholar]
- Release, S. 3: Desmond Molecular Dynamics System; Maestro-Desmond Interoperability Tools; DE Shaw Research: New York, NY, USA; Schrödinger: New York, NY, USA, 2017. [Google Scholar]
- Phillips, J.; Braun, R.; Wang, W.; Gumbart, J.; Tajkhorshid, E.; Villa, E.; Chipot, C.; Skeel, R.; Kalé, L.; Schulten, K. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005, 26, 1781–1802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Oshima, H.; Zhang, H.; Kern, N.; Re, S.; Lee, J.; Roux, B.; Sugita, Y.; Jiang, W.; Im, W. CHARMM-GUI free energy calculator for absolute and relative ligand solvation and binding free energy simulations. J. Chem. Theory Comput. 2020, 16, 7207–7218. [Google Scholar] [CrossRef] [PubMed]
- Ngo, S.T.; Tam, N.M.; Pham, M.Q.; Nguyen, T.H. Benchmark of popular free energy approaches revealing the inhibitors binding to SARS-CoV-2 Mpro. J. Chem. Inf. Model. 2021, 61, 2302–2312. [Google Scholar] [CrossRef] [PubMed]















| No. | Identified Compound | Ionization Mode | m/z | M.F. | Accurate Mass | Calculated Mass | Chemical Class |
|---|---|---|---|---|---|---|---|
| 1 | Apigenin | Positive | 271.0606 | C15H10O5 | 270.0529 | 270.0528 | Flavonoids |
| 2 | Kaempferol | Positive | 287.0557 | C15H10O6 | 286.0479 | 286.0477 | Flavonoids |
| 3 | Quercetin | Positive | 303.0508 | C15H10O7 | 302.0430 | 302.0427 | Flavonoids |
| 4 | Populnin | Positive | 449.103 | C21H20O11 | 448.1001 | 448.1006 | Flavonoids |
| 5 | Kaempferol 3-rutinoside-7-glucoside | Positive | 757.2194 | C33H40O20 | 756.2116 | 756.2113 | Flavonoids |
| 6 | Quercetin 3-rutinoside-7-glucoside | Positive | 773.2138 | C33H40O20 | 772.2060 | 772.2062 | Flavonoids |
| 7 | Pelargonidin 3-glucoside | Positive | 433.1126 | C21H21O10 | 433.1126 | 433.1129 | Anthocyanin |
| 8 | Cyanidin 3-glucoside | Positive | 449.1080 | C21H21O11 | 449.1080 | 449.1078 | Anthocyanin |
| 9 | Ferulic acid | Negative | 193.0496 | C10H10O4 | 194.0574 | 194.0579 | Phenolic acids |
| 10 | 1-O-Cinnamoyl-beta-D-glucose | Negative | 309.0971 | C15H18O7 | 310.1050 | 310.1053 | Phenolic acids |
| 11 | Protocatechuic acid | Negative | 155.0347 | C7H6O4 | 154.0269 | 154.0266 | Phenolic acids |
| 12 | Benzoic acid | Negative | 123.0444 | C7H6O2 | 122.0366 | 122.0368 | Phenolic acids |
| 13 | Morusimic acid A | Negative | 490.3015 | C24H45NO9 | 491.3095 | 491.3094 | Alkaloid |
| Formula Number | AgNO3(g) | Pectin (g) | Particle Size (nm) | PDI | EE% | Z-Potential (mv) |
|---|---|---|---|---|---|---|
| 1 | 0.104 | 0.1 | 190.3 ± 5.6 | 0.35 ± 0.01 | 41.8 ± 3.2 | −25.6 |
| 2 | 0.2 | 0.4 | 37.2 ± 1.3 | 0.37 ± 0.02 | 39.3 ± 2.5 | −32.5 |
| 3 | 0.05 | 0.209 | 248.9 ± 12.5 | 0.59 ± 0.005 | 37.5 ± 5.4 | −27.6 |
| 4 | 0.0725 | 0.4 | 300.8 ± 6.5 | 0.40 ± 0.007 | 38.1 ± 1.2 | −30.4 |
| 5 | 0.2 | 0.145 | 220.2 ± 10.6 | 0.36 ± 0.01 | 40.6 ± 2.9 | −25.3 |
| Degenerative Changes | Dilated and Congested Central Vein | Dilated Sinusoids | Hepatocytes Necrosis | Vacuolar Degenerations | Inflammatory Cellular Infiltrates |
|---|---|---|---|---|---|
| Sham | 0 | 0 | 0 | 0 | 0 |
| IRI | 3 | 3 | 3 | 3 | 3 |
| BBE | 2 | 1 | 1 | 2 | 2 |
| AgNPs | 2 | 2 | 2 | 2 | 2 |
| 200 BBE-AgNPs | 2 | 1 | 0 | 1 | 1 |
| 50 BBE-AgNPs | 1 | 1 | 0 | 1 | 0 |
| Silymarin | 0 | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fathi, A.M.; Waz, S.; Alaaeldin, E.; Toni, N.D.M.; El-Sheikh, A.A.K.; Sayed, A.M.; Abdelmohsen, U.R.; Nazmy, M.H. Blackberry-Loaded AgNPs Attenuate Hepatic Ischemia/Reperfusion Injury via PI3K/Akt/mTOR Pathway. Metabolites 2023, 13, 419. https://doi.org/10.3390/metabo13030419
Fathi AM, Waz S, Alaaeldin E, Toni NDM, El-Sheikh AAK, Sayed AM, Abdelmohsen UR, Nazmy MH. Blackberry-Loaded AgNPs Attenuate Hepatic Ischemia/Reperfusion Injury via PI3K/Akt/mTOR Pathway. Metabolites. 2023; 13(3):419. https://doi.org/10.3390/metabo13030419
Chicago/Turabian StyleFathi, Ahmed M., Shaimaa Waz, Eman Alaaeldin, Nisreen D. M. Toni, Azza A. K. El-Sheikh, Ahmed M. Sayed, Usama Ramadan Abdelmohsen, and Maiiada H. Nazmy. 2023. "Blackberry-Loaded AgNPs Attenuate Hepatic Ischemia/Reperfusion Injury via PI3K/Akt/mTOR Pathway" Metabolites 13, no. 3: 419. https://doi.org/10.3390/metabo13030419
APA StyleFathi, A. M., Waz, S., Alaaeldin, E., Toni, N. D. M., El-Sheikh, A. A. K., Sayed, A. M., Abdelmohsen, U. R., & Nazmy, M. H. (2023). Blackberry-Loaded AgNPs Attenuate Hepatic Ischemia/Reperfusion Injury via PI3K/Akt/mTOR Pathway. Metabolites, 13(3), 419. https://doi.org/10.3390/metabo13030419