Influence of Coronary Artery Bypass Grafts on Blood Aminothiols in Patients with Coronary Artery Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Characteristics
2.2. Laboratory Studies
2.3. Determination of bGSH, GSSG, and RS GSH
2.4. Determination of Aminothiols in Plasma
2.5. Determination of SAM and SAH in Plasma
2.6. Data Processing
3. Results
4. Discussion
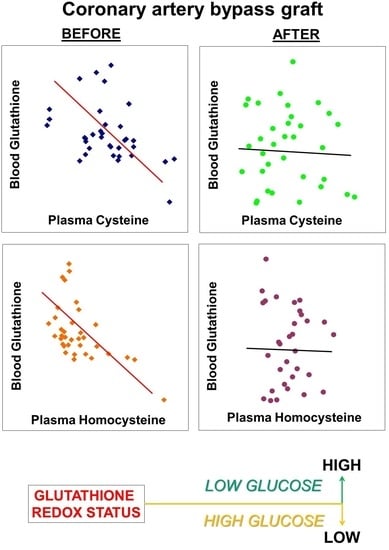
4.1. Impacts of Aminothiols and Glucose on GSH Metabolism in CAD
4.2. Impact of CABG on Aminothiols
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef] [Green Version]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Simoons, M.L.; Chaitman, B.R.; White, H.D.; Katus, H.A.; Apple, F.S.; Lindahl, B.; Morrow, D.A.; et al. Writing Group on the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction. Third universal definition of myocardial infarction. Eur. Heart J. 2012, 33, 2551–2567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varadhan, S.; Venkatachalam, R.; Perumal, S.M.; Ayyamkulamkara, S.S. Evaluation of Oxidative Stress Parameters and Antioxidant Status in Coronary Artery Disease Patients. Arch. Razi Inst. 2022, 77, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Bastani, A.; Rajabi, S.; Daliran, A.; Saadat, H.; Karimi-Busheri, F. Oxidant and antioxidant status in coronary artery disease. Biomed. Rep. 2018, 9, 327–332. [Google Scholar] [CrossRef] [Green Version]
- Caussé, E.; Fournier, P.; Roncalli, J.; Salvayre, R.; Galinier, M. Serum allantoin and aminothiols as biomarkers of chronic heart failure. Acta Cardiol. 2017, 72, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Dogan, A.; Turker, F.S. The Effect of On-Pump and Off-Pump Bypass Operations on Oxidative Damage and Antioxidant Parameters. Oxid. Med. Cell. Longev. 2017, 2017, 8271376. [Google Scholar] [CrossRef] [Green Version]
- Cavalca, V.; Sisillo, E.; Veglia, F.; Tremoli, E.; Cighetti, G.; Salvi, L.; Sola, A.; Mussoni, L.; Biglioli, P.; Folco, G.; et al. Isoprostanes and oxidative stress in off-pump and on-pump coronary bypass surgery. Ann. Thorac. Surg. 2006, 81, 562–567. [Google Scholar] [CrossRef]
- Zakkar, M.; Guida, G.; Suleiman, M.S.; Angelini, G.D. Cardiopulmonary bypass and oxidative stress. Oxid. Med. Cell. Longev. 2015, 2015, 189863. [Google Scholar] [CrossRef]
- Oral, H. Post-operative atrial fibrillation and oxidative stress: A novel causal mechanism or another biochemical epiphenomenon? J. Am. Coll. Cardiol. 2008, 51, 75–76. [Google Scholar] [CrossRef] [Green Version]
- Gwozdzinski, K.; Pieniazek, A.; Bernasinska-Slomczewska, J.; Brzeszczynska, J.; Irzmanski, R.; Jegier, A. Alterations in the Properties of Red Blood Cells in Men with Coronary Artery Diseases after Comprehensive Cardiac Rehabilitation. Cardiol. Res. Pract. 2020, 2020, 6478785. [Google Scholar] [CrossRef]
- Schuh, A.K.; Sheybani, B.; Jortzik, E.; Niemann, B.; Wilhelm, J.; Boening, A.; Becker, K. Redox status of patients before cardiac surgery. Redox Rep. 2018, 23, 83–93. [Google Scholar] [CrossRef]
- Asensi, M.; Sastre, J.; Pallardo, F.V.; Lloret, A.; Lehner, M.; Garcia-de-la Asuncion, J.; Viña, J. Ratio of reduced to oxidized glutathione as indicator of oxidative stress status and DNA damage. Methods Enzymol. 1999, 299, 267–276. [Google Scholar] [CrossRef]
- Lu, S.C. Regulation of glutathione synthesis. Mol. Asp. Med. 2009, 30, 42–59. [Google Scholar] [CrossRef] [Green Version]
- Williams, R.H.; Maggiore, J.A.; Reynolds, R.D.; Helgason, C.M. Novel approach for the determination of the redox status of homocysteine and other aminothiols in plasma from healthy subjects and patients with ischemic stroke. Clin. Chem. 2001, 47, 1031–1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karolczak, K.; Kubalczyk, P.; Głowacki, R.; Pietruszyński, R.; Watała, C. An inverse relationship between plasma glutathione concentration and fasting glycemia in patients with coronary artery disease and concomitant type 2 diabetes: A pilot study. Adv. Clin. Exp. Med. 2017, 26, 1359–1366. [Google Scholar] [CrossRef] [Green Version]
- Du, S.; Shi, H.; Xiong, L.; Wang, P.; Shi, Y. Canagliflozin mitigates ferroptosis and improves myocardial oxidative stress in mice with diabetic cardiomyopathy. Front. Endocrinol. 2022, 13, 1011669. [Google Scholar] [CrossRef] [PubMed]
- Svarovskaya, A.V.; Arzhanik, M.B.; Ogurkova, O.N.; Kuzheleva, E.A.; Baev, A.E.; Garganeeva, A.A. Predictive value of laboratory markers in the development of cardiac events in patients with stable coronary artery disease after elective endovascular revascularization. Kardiologiia 2021, 61, 33–39. [Google Scholar] [CrossRef]
- Kim, S.J.; Song, P.; Park, J.H.; Lee, Y.T.; Kim, W.S.; Park, Y.G.; Bang, O.Y.; Chung, C.S.; Lee, K.H.; Kim, G.M. Biomarkers of asymptomatic carotid stenosis in patients undergoing coronary artery bypass grafting. Stroke 2011, 42, 734–739. [Google Scholar] [CrossRef] [Green Version]
- Balogh, E.; Maros, T.; Daragó, A.; Csapó, K.; Herceg, B.; Nyul, B.; Czuriga, I.; Bereczky, Z.; Édes, I.; Koszegi, Z. Plasma homocysteine levels are related to medium-term venous graft degeneration in coronary artery bypass graft patients. Anatol. J. Cardiol. 2016, 16, 868–873. [Google Scholar] [CrossRef]
- Ranucci, M.; Ballotta, A.; Frigiola, A.; Boncilli, A.; Brozzi, S.; Costa, E.; Mehta, R.H. Pre-operative homocysteine levels and morbidity and mortality following cardiac surgery. Eur. Heart J. 2009, 30, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- Jeremy, J.Y.; Shukla, N.; Angelini, G.D.; Day, A.; Wan, I.Y.; Talpahewa, S.P.; Ascione, R. Sustained increases of plasma homocysteine, copper, and serum ceruloplasmin after coronary artery bypass grafting. Ann. Thorac. Surg. 2002, 74, 1553–1557. [Google Scholar] [CrossRef]
- Thiengburanatham, S. Hyperhomocysteinemia-induced myocardial injury after coronary artery bypass. Asian Cardiovasc. Thorac. Ann. 2009, 17, 483–489. [Google Scholar] [CrossRef]
- Go, Y.M.; Jones, D.P. Cysteine/cystine redox signaling in cardiovascular disease. Free Radic. Biol. Med. 2011, 50, 495–509. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, S.; Shino, M.; Fujikawa, T.; Itoh, Y.; Ueda, E.; Hashimoto, T.; Kuji, T.; Kobayashi, N.; Ohnishi, T.; Hirawa, N.; et al. Plasma Cystine Levels and Cardiovascular and All-Cause Mortality in Hemodialysis Patients. Ther. Apher. Dial. 2018, 22, 476–484. [Google Scholar] [CrossRef]
- Pereira, E.C.; Bertolami, M.C.; Faludi, A.A.; Monte, O.; Xavier, H.T.; Pereira, T.V.; Abdalla, D.S. Predictive Potential of Twenty-Two Biochemical Biomarkers for Coronary Artery Disease in Type 2 Diabetes Mellitus. Int. J. Endocrinol. 2015, 2015, 146816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavalca, V.; Tremoli, E.; Porro, B.; Veglia, F.; Myasoedova, V.; Squellerio, I.; Manzone, D.; Zanobini, M.; Trezzi, M.; Di Minno, M.N.; et al. Oxidative stress and nitric oxide pathway in adult patients who are candidates for cardiac surgery: Patterns and differences. Interact. Cardiovasc. Thorac. Surg. 2013, 17, 923–930. [Google Scholar] [CrossRef] [Green Version]
- Patel, R.S.; Ghasemzadeh, N.; Eapen, D.J.; Sher, S.; Arshad, S.; Ko, Y.A.; Veledar, E.; Samady, H.; Zafari, A.M.; Sperling, L.; et al. Novel Biomarker of Oxidative Stress Is Associated with Risk of Death in Patients with Coronary Artery Disease. Circulation 2016, 133, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Pietruszyński, R.; Markuszewski, L.; Masiarek, K.; Makowski, M.; Retelewska, W.; Watala, C. Role of preprocedural glutathione concentrations in the prediction of major adverse cardiac events in patients with acute coronary syndrome treated with percutaneous coronary intervention. Pol. Arch. Med. Wewn. 2013, 123, 228–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matuz-Mares, D.; Riveros-Rosas, H.; Vilchis-Landeros, M.M.; Vázquez-Meza, H. Glutathione Participation in the Prevention of Cardiovascular Diseases. Antioxidants 2021, 10, 1220. [Google Scholar] [CrossRef]
- Ivanov, A.V.; Alexandrin, V.V.; Paltsyn, A.A.; Virus, E.D.; Nikiforova, K.A.; Bulgakova, P.O.; Sviridkina, N.B.; Apollonova, S.A.; Kubatiev, A.A. Metoprolol and Nebivolol Prevent the Decline of the Redox Status of Low-Molecular-Weight Aminothiols in Blood Plasma of Rats During Acute Cerebral Ischemia. J. Cardiovasc. Pharmacol. 2018, 72, 195–203. [Google Scholar] [CrossRef]
- Human IL-6. Platinum ELISA. In Product Information & Manual; Bender MedSystems GmbH Campus Vienna Biocenter 2: Vienna, Austria, 2012; Available online: http://www.ulab360.com/files/prod/manuals/201305/15/376695001.pdf (accessed on 2 June 2023).
- Ivanov, A.V.; Popov, M.A.; Aleksandrin, V.V.; Kozhevnikova, L.M.; Moskovtsev, A.A.; Kruglova, M.P.; Vladimirovna, S.E.; Aleksandrovich, S.V.; Kubatiev, A.A. Determination of glutathione in blood via capillary electrophoresis with pH-mediated stacking. Electrophoresis 2022, 43, 1859–1870. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.V.; Dubchenko, E.A.; Kruglova, M.P.; Virus, E.D.; Bulgakova, P.O.; Alexandrin, V.V.; Fedoseev, A.N.; Boyko, A.N.; Grachev, S.V.; Kubatiev, A.A. Determination of S-adenosylmethionine and S-adenosylhomocysteine in blood plasma by UPLC with fluorescence detection. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2019, 1124, 366–374. [Google Scholar] [CrossRef]
- Hu, H.; Chen, Y.; Jing, L.; Zhai, C.; Shen, L. The Link between Ferroptosis and Cardiovascular Diseases: A Novel Target for Treatment. Front. Cardiovasc. Med. 2021, 8, 710963. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, H.; Hua, L.; Hou, C.; Jia, Q.; Chen, J.; Zhang, S.; Wang, Y.; He, S.; Jia, E. Verification of ferroptosis and pyroptosis and identification of PTGS2 as the hub gene in human coronary artery atherosclerosis. Free Radic. Biol. Med. 2021, 171, 55–68. [Google Scholar] [CrossRef]
- Tadokoro, T.; Ikeda, M.; Ide, T.; Deguchi, H.; Ikeda, S.; Okabe, K.; Ishikita, A.; Matsushima, S.; Koumura, T.; Yamada, K.I.; et al. Mitochondria-dependent ferroptosis plays a pivotal role in doxorubicin cardiotoxicity. JCI Insight 2020, 5, e132747. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zheng, M.Q.; Rozanski, G.J. Glutathione homeostasis in ventricular myocytes from rat hearts with chronic myocardial infarction. Exp. Physiol. 2009, 94, 815–824. [Google Scholar] [CrossRef]
- Valerio, V.; Myasoedova, V.A.; Moschetta, D.; Porro, B.; Perrucci, G.L.; Cavalca, V.; Cavallotti, L.; Songia, P.; Poggio, P. Impact of Oxidative Stress and Protein S-Glutathionylation in Aortic Valve Sclerosis Patients with Overt Atherosclerosis. J. Clin. Med. 2019, 8, 552. [Google Scholar] [CrossRef] [Green Version]
- Musthafa, Q.A.; Abdul Shukor, M.F.; Ismail, N.A.S.; Mohd Ghazi, A.; Mohd Ali, R.; M Nor, I.F.; Dimon, M.Z.; Wan Ngah, W.Z. Oxidative status and reduced glutathione levels in premature coronary artery disease and coronary artery disease. Free Radic. Res. 2017, 51, 787–798. [Google Scholar] [CrossRef]
- Palazhy, S.; Kamath, P.; Vasudevan, D.M. Elevated oxidative stress among coronary artery disease patients on statin therapy: A cross sectional study. Indian Heart J. 2015, 67, 227–232. [Google Scholar] [CrossRef] [Green Version]
- Boneberg, R.; Pardun, A.; Hannemann, L.; Hildebrandt, O.; Koehler, U.; Kinscherf, R.; Hildebrandt, W. High Plasma Cystine Levels Are Associated with Blood Pressure and Reversed by CPAP in Patients with Obstructive Sleep Apnea. J. Clin. Med. 2021, 10, 1387. [Google Scholar] [CrossRef]
- Ye, Y.; Chen, A.; Li, L.; Liang, Q.; Wang, S.; Dong, Q.; Fu, M.; Lan, Z.; Li, Y.; Liu, X.; et al. Repression of the antiporter SLC7A11/glutathione/glutathione peroxidase 4 axis drives ferroptosis of vascular smooth muscle cells to facilitate vascular calcification. Kidney Int. 2022, 102, 1259–1275. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Cai, Z.; Wang, H.; Han, D.; Cheng, Q.; Zhang, P.; Gao, F.; Yu, Y.; Song, Z.; Wu, Q.; et al. Loss of Cardiac Ferritin H Facilitates Cardiomyopathy via Slc7a11-Mediated Ferroptosis. Circ. Res. 2020, 127, 486–501. [Google Scholar] [CrossRef]
- Liu, L.; He, J.; Sun, G.; Huang, N.; Bian, Z.; Xu, C.; Zhang, Y.; Cui, Z.; Xu, W.; Sun, F.; et al. The N6-methyladenosine modification enhances ferroptosis resistance through inhibiting SLC7A11 mRNA deadenylation in hepatoblastoma. Clin. Transl. Med. 2022, 12, e778. [Google Scholar] [CrossRef]
- Granitzer, S.; Widhalm, R.; Forsthuber, M.; Ellinger, I.; Desoye, G.; Hengstschläger, M.; Zeisler, H.; Salzer, H.; Gundacker, C. Amino Acid Transporter LAT1 (SLC7A5) Mediates MeHg-Induced Oxidative Stress Defense in the Human Placental Cell Line HTR-8/SVneo. Int. J. Mol. Sci. 2021, 22, 1707. [Google Scholar] [CrossRef]
- Jersin, R.Å.; Jonassen, L.R.; Dankel, S.N. The neutral amino acid transporter SLC7A10 in adipose tissue, obesity and insulin resistance. Front. Cell Dev. Biol. 2022, 10, 974338. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, J.D. Methionine metabolism in mammals. J. Nutr. Biochem. 1990, 1, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Nandi, S.S.; Mishra, P.K. H2S and homocysteine control a novel feedback regulation of cystathionine beta synthase and cystathionine gamma lyase in cardiomyocytes. Sci. Rep. 2017, 7, 3639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.J.; Li, Q.; Du, H.P.; Wang, Y.L.; You, S.J.; Wang, F.; Xu, X.S.; Cheng, J.; Cao, Y.J.; Liu, C.F.; et al. Homocysteine Triggers Inflammatory Responses in Macrophages through Inhibiting CSE-H2S Signaling via DNA Hypermethylation of CSE Promoter. Int. J. Mol. Sci. 2015, 16, 12560–12577. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; He, G.W. Imbalance of Homocysteine and H2S: Significance, Mechanisms, and Therapeutic Promise in Vascular Injury. Oxid. Med. Cell. Longev. 2019, 2019, 7629673. [Google Scholar] [CrossRef] [Green Version]
- Nakano, S.; Ishii, I.; Shinmura, K.; Tamaki, K.; Hishiki, T.; Akahoshi, N.; Ida, T.; Nakanishi, T.; Kamata, S.; Kumagai, Y.; et al. Hyperhomocysteinemia abrogates fasting-induced cardioprotection against ischemia/reperfusion by limiting bioavailability of hydrogen sulfide anions. J. Mol. Med. 2015, 93, 879–889. [Google Scholar] [CrossRef]
- Kimura, Y.; Kimura, H. Hydrogen sulfide protects neurons from oxidative stress. FASEB J. 2004, 18, 1165–1167. [Google Scholar] [CrossRef]
- Yildiz, D.; Ekin, S.; Sahinalp, S. Evaluations of Antioxidant Enzyme Activities, Total Sialic Acid and Trace Element Levels in Coronary Artery Bypass Grafting Patients. Braz. J. Cardiovasc. Surg. 2021, 36, 769–779. [Google Scholar] [CrossRef]
- Iqbal, M.P.; Ishaq, M.; Mehboobali, N. Increased levels of erythrocyte glutathione in acute myocardial infarction: An antioxidant defence. J. Pak. Med. Assoc. 2004, 54, 254–258. [Google Scholar] [PubMed]
- Wang, C.; Zhu, L.; Yuan, W.; Sun, L.; Xia, Z.; Zhang, Z.; Yao, W. Diabetes aggravates myocardial ischaemia reperfusion injury via activating Nox2-related programmed cell death in an AMPK-dependent manner. J. Cell. Mol. Med. 2020, 24, 6670–6679. [Google Scholar] [CrossRef] [PubMed]
- Luo, E.F.; Li, H.X.; Qin, Y.H.; Qiao, Y.; Yan, G.L.; Yao, Y.Y.; Li, L.Q.; Hou, J.T.; Tang, C.C.; Wang, D. Role of ferroptosis in the process of diabetes-induced endothelial dysfunction. World J. Diabetes 2021, 12, 124–137. [Google Scholar] [CrossRef]
- Akila; D’souza, B.; Vishwanath, P.; D’souza, V. Oxidative injury and antioxidants in coronary artery bypass graft surgery: Off-pump CABG significantly reduces oxidative stress. Clin. Chim. Acta 2007, 375, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Biagioli, B.; Borrelli, E.; Maccherini, M.; Bellomo, G.; Lisi, G.; Giomarelli, P.; Sani, G.; Toscano, M. Reduction of oxidative stress does not affect recovery of myocardial function: Warm continuous versus cold intermittent blood cardioplegia. Heart 1997, 77, 465–473. [Google Scholar] [CrossRef]
- Karu, I.; Loit, R.; Paapstel, A.; Kairane, C.; Zilmer, M.; Starkopf, J. Early postoperative function of the heart after coronary artery bypass grafting is not predicted by myocardial necrosis and glutathione-associated oxidative stress. Clin. Chim. Acta 2005, 359, 195–202. [Google Scholar] [CrossRef]
- Palomero, J.; Galán, A.I.; Muñoz, M.E.; González-Gallego, J.; Tuñón, M.J.; Jiménez, R. S-adenosylmethionine protects against intrabiliary glutathione degradation induced by long-term administration of cyclosporin A in the rat. Toxicology 2004, 201, 239–245. [Google Scholar] [CrossRef]
- Kilanczyk, E.; Banales, J.M.; Wunsch, E.; Barbier, O.; Avila, M.A.; Mato, J.M.; Milkiewicz, M.; Milkiewicz, P. S-adenosyl-L-methionine (SAMe) halts the autoimmune response in patients with primary biliary cholangitis (PBC) via antioxidant and S-glutathionylation processes in cholangiocytes. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165895. [Google Scholar] [CrossRef]
- Kryukov, E.V.; Ivanov, A.V.; Karpov, V.O.; Vasil’evich Aleksandrin, V.; Dygai, A.M.; Kruglova, M.P.; Kostiuchenko, G.I.; Kazakov, S.P.; Kubatiev, A.A. Plasma S-Adenosylmethionine Is Associated with Lung Injury in COVID-19. Dis. Markers 2021, 2021, 7686374. [Google Scholar] [CrossRef] [PubMed]
- Semmler, A.; Smulders, Y.; Struys, E.; Smith, D.; Moskau, S.; Blom, H.; Linnebank, M. Methionine metabolism in an animal model of sepsis. Clin. Chem. Lab. Med. 2008, 46, 1398–1402. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| CAD Group (N = 35) | Control Group (N = 43) | p | |
|---|---|---|---|
| Sex: M (%)/F (%) | 23 (66%)/12 (34%) | 22 (51%)/21 (49%) | NS # |
| Age (y) | 59 [55; 63.5] | 57 [52; 63.5] | NS * |
| CABG | On-pump: 10 (29%) Off-pump: 25 (71%) | - | |
| Hypertension: Yes (%)/No (%) | 35 (100%)/0 (0%) 2nd stage: 10, 3rd stage: 25 | 0 (0%)/43 (100%) | <0.001 # |
| DM: Yes (%)/No (%) | 17 (49%)/18 (51%) | 0 (0%)/43 (100%) | <0.001 # |
| Fibrillation: Yes (%)/No (%) | 21 (60%)/14 (40%) | 0 (0%)/43 (100%) | <0.001 # |
| Infarct: Yes (%)/No (%) | 25 (71%)/10 (29%) | 0 (0%)/43 (100%) | <0.001 # |
| Smoke: Yes (%)/No (%) | 21 (60%)/14 (40%) | 25 (58%)/18 (42%) | NS # |
| Diet 1: Yes (%)/No (%) | 8 (23%)/27 (77%) | 10 (23%)/33 (77%) | NS # |
| Blood loss (mL) | 650 (min 500, max 700) | - | |
| Functional class according to NYHA $ | II: 23 (66%) III: 12 (34%) | - | |
| Atherogenic coefficient | - | ||
| Normal (<3.5) | 16 (46%) | ||
| Elevated (>4) | 19 (54%) | ||
| Left ventricular ejection fraction (%) | 55 [52; 58] | - | |
| Obesity | 4 (11%) | - | |
| Hyperlipidemia | 2 (6%) | - | |
| Glucose (mM) | 4.5 [4.1; 4.9] | - | |
| Cholesterol (mM) | 4.4 [3.65; 4.85] | - | |
| LDL Cholesterol (mM) | 2.5 [2.2; 3.1] | - | |
| HDL Cholesterol (mM) | 1.1 [0.9; 1.4] | - | |
| HHcy (tHcy > 15 mkM) | 27 (77%) | 3 (7%) | <0.001 # |
| Indicator | Preoperative Level | Postoperative Level | p a |
|---|---|---|---|
| HCT (%) | 40 [38; 41] | 40 [38; 40] | NS |
| RBC (106/μL) | 4.9 [4.7; 5.3] | 3.8 [3.7; 3.9] | <0.001 |
| Hb (g/L) | 140 [136; 145] | 99 [93; 104] | <0.001 |
| MCV (fL) | 89 [87; 96.5] | 92 [88; 97.5] | NS |
| WBC (103/μL) | 7 [5.5; 7.0] | 20 [16; 22] | <0.001 |
| PLT (103/μL) | 259 [211; 311] | 154 [127; 195] | <0.001 |
| PATT (sec) | 32 [29; 34] | 26 [25; 28] | <0.001 |
| CRP (mg/L) | 4 [3; 6] | 24 [20; 34.5] | <0.001 |
| Fibrinogen (g/L) | 3.9 [3.7; 4.1] | 2.7 [2.3; 3.0] | <0.001 |
| Ferritin (μg/L) | 68 [49; 83.5] | 190 [169; 295] | <0.001 |
| IL-6 (pg/mL) | 5 [3; 6] | 19 [14; 24] | <0.001 |
| Indicator | Controls | p a | Preoperative Level | p b | Postoperative Level |
|---|---|---|---|---|---|
| tCys (μM) | 286 [277; 299] | <0.001 | 331 [294; 368] | NS | 290 [244; 330] |
| tCG (μM) | 31.1 [27.5; 35.4] | <0.001 | 23.5 [21; 29] | NS | 24.7 [22.1; 29.8] |
| tGSH (μM) | 10.8 [8.6; 11.9] | <0.001 | 20.5 [16.6; 23.8] | NS | 16.6 [13.2; 19.0] |
| tHcy (μM) | 12.3 [11.6; 13.3] | <0.001 | 18.2 [15.3; 22.2] | NS | 17.9 [14.4; 20.4] |
| SAM (nM) | 104 [91; 119] | <0.001 | 72 [62; 94] | NS | 79 [73; 96] |
| SAH (nM) | 9.4 [7.3; 11.6] | NS | 11.1 [7.1; 15.7] | NS | 15.2 [9.4; 17.9] |
| SAM/SAH | 11.0 [9.0; 13.2] | <0.001 | 6.0 [4.0; 9.2] | NS | 5.8 [4.5; 7.8] |
| bGSH (μM) | 968 [876; 1028] | <0.001 | 755 [682; 842] | 0.005 | 698 [554; 802] |
| GSSG (μM) | 3.54 [2.80; 4.23] | >0.05 | 3.9 [3.1; 4.8] | NS | 3.0 [2.2; 6.5] |
| RS GSH | 545 [466; 647] | <0.001 | 408 [339; 484] | NS | 416 [269; 532] |
| bGSH/tCys | 3.41 [2.93; 3.70] | <0.001 | 2.36 [1.93; 2.64] | NS | 2.39 [1.93; 2.90] |
| bGSH/Hb (μmol/g) | - | - | 5.45 [4.74; 6.04] | <0.001 | 7.21 [5.55; 8.34] |
| Indicators | Controls | CAD | CABG | |||
|---|---|---|---|---|---|---|
| R * | p | R * | p | R * | p | |
| bGSH and GSSG | 0.607 | 0.002 | 0.354 | NS | 0.773 | <0.001 |
| bGSH and tCys | −0.538 | 0.011 | −0.429 | 0.043 | 0.097 | NS |
| bGSH/Hb and tCys | - | - | −0.415 | 0.045 | 0.091 | NS |
| bGSH and tHcy | 0.217 | NS | −0.545 | 0.018 | 0.006 | NS |
| bGSH/Hb and tHcy | - | - | −0.531 | 0.025 | 0.093 | NS |
| bGSH and SAH | −0.563 | 0.005 | −0.078 | NS | 0.225 | NS |
| GSSG and tCys | −0.551 | 0.011 | −0.275 | NS | 0.213 | NS |
| tCys and tCG | 0.372 | NS | 0.505 | 0.043 | 0.354 | >0.05 |
| tCys and tHcy | 0.214 | NS | 0.743 | <0.001 | 0.354 | NS |
| tCys and SAM | 0.049 | NS | 0.707 | <0.001 | −0.045 | NS |
| tCys and SAH | 0.199 | NS | 0.565 | 0.018 | 0.117 | NS |
| tCys and tGSH | −0.484 | 0.028 | 0.150 | NS | 0.067 | NS |
| tHcy and SAM | −0.27 | NS | 0.546 | 0.019 | 0.375 | NS |
| tHcy and SAH | −0.078 | NS | 0.664 | 0.001 | 0.462 | NS |
| tGSH and SAM | −0.181 | NS | 0.218 | NS | −0.522 | 0.042 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanov, A.V.; Popov, M.A.; Metelkin, A.A.; Aleksandrin, V.V.; Agafonov, E.G.; Kruglova, M.P.; Silina, E.V.; Stupin, V.A.; Maslennikov, R.A.; Kubatiev, A.A. Influence of Coronary Artery Bypass Grafts on Blood Aminothiols in Patients with Coronary Artery Disease. Metabolites 2023, 13, 743. https://doi.org/10.3390/metabo13060743
Ivanov AV, Popov MA, Metelkin AA, Aleksandrin VV, Agafonov EG, Kruglova MP, Silina EV, Stupin VA, Maslennikov RA, Kubatiev AA. Influence of Coronary Artery Bypass Grafts on Blood Aminothiols in Patients with Coronary Artery Disease. Metabolites. 2023; 13(6):743. https://doi.org/10.3390/metabo13060743
Chicago/Turabian StyleIvanov, Alexander Vladimirovich, Mikhail Aleksandrovich Popov, Arkady Andreevich Metelkin, Valery Vasil’evich Aleksandrin, Evgeniy Gennad’evich Agafonov, Maria Petrovna Kruglova, Ekaterina Vladimirovna Silina, Victor Aleksandrovich Stupin, Ruslan Andreevich Maslennikov, and Aslan Amirkhanovich Kubatiev. 2023. "Influence of Coronary Artery Bypass Grafts on Blood Aminothiols in Patients with Coronary Artery Disease" Metabolites 13, no. 6: 743. https://doi.org/10.3390/metabo13060743
APA StyleIvanov, A. V., Popov, M. A., Metelkin, A. A., Aleksandrin, V. V., Agafonov, E. G., Kruglova, M. P., Silina, E. V., Stupin, V. A., Maslennikov, R. A., & Kubatiev, A. A. (2023). Influence of Coronary Artery Bypass Grafts on Blood Aminothiols in Patients with Coronary Artery Disease. Metabolites, 13(6), 743. https://doi.org/10.3390/metabo13060743