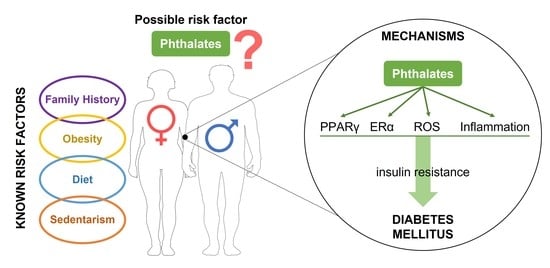
The Relationship between Phthalates and Diabetes: A Review
Abstract
:1. Introduction
2. Phthalates
3. Diabetes Mellitus
3.1. Gestational Diabetes Mellitus
3.2. Type 1 Diabetes Mellitus
3.3. Type 2 Diabetes Mellitus
4. Phthalates as a Risk Factor for Diabetes Mellitus
4.1. Gestational Diabetes Mellitus
4.1.1. Epidemiological Studies
4.1.2. Experimental Studies
4.1.3. Possible Mechanisms
4.2. Type 1 Diabetes Mellitus
4.2.1. Epidemiological Studies
4.2.2. Experimental Studies
4.2.3. Possible Mechanisms
4.3. Type 2 Diabetes Mellitus
4.3.1. Epidemiological Studies
4.3.2. Experimental Studies
4.3.3. Possible Mechanisms
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ∑DBP | Sum of DBP metabolites |
| ∑DEHP | Sum of DEHP metabolites |
| 1.1B4 | Human pancreatic β-cells |
| 8-OHdG | 8-hydroxy-2′-deoxyguanosine |
| 8-PGF2α | 8-iso-prostaglandin F2α |
| ATM | Ataxia-telangiectasia mutated |
| Bax | Bcl-2-associated X protein |
| BBzP | Butylbenzyl phthalate |
| Bcl-2 | B-cell lymphoma 2 anti-apoptotic protein |
| BMI | Body mass index |
| BPA | Bisphenol-A |
| DBP | Di-butyl phthalate |
| DEHP | Di-(2-ethylhexyl) phthalate |
| DEP | Diethyl phthalate |
| DiBP | Di-isobutyl phthalate |
| DiDP | Diisodecyl phthalate |
| DiNP | Diisononyl phthalate |
| DMP | Dimethyl phthalate |
| DMAQ-B1 | Demethylasterriquinone B1 |
| DnOP | Di-n-octyl phthalate |
| DPHP | Di(2-propylheptyl) phthalate |
| EDC | Endocrine disruptor compound |
| ER | Estrogen receptors |
| FoxM1 | Forkhead box protein M1 |
| GD | Gestational day |
| GDM | Gestational diabetes mellitus |
| GLUT | Glucose transporter protein |
| GWG | Gestational weight gain |
| HbA1c | Glycosylated hemoglobin |
| HMW | High molecular weight phthalates |
| HOMA-IR | Homeostasis model assessment-estimated insulin resistance |
| IDF | International diabetes federation |
| INS-1E | Rat pancreatic β-cell line |
| JNK | Jun-N-terminal kinase |
| LMW | Low molecular weight phthalates |
| MBP | Mono-n-butyl phthalate |
| MBzP | Mono-benzyl phthalate |
| MCMHP | Mono-(2-carboxymethyl-hexyl) phthalate |
| MCNP | Mono-(carboxy-isononyl) phthalate |
| MCOP | Mono-(carboxy-isooctyl) phthalate |
| MCPP | Mono-(3-carboxypropyl) phthalate |
| MDA | Malondialdehyde |
| MECPP | Mono-(2-ethyl-5-carboxypentyl) phthalate |
| MECPTP | Mono-2-ethyl-5-carboxypentyl terephthalate |
| MEHHP | Mono-(2-ethyl-5-hydroxyhexyl) phthalate |
| MEHP | Mono-(2-ethylhexyl) phthalate |
| MEOHP | Mono-(2-ethyl-5-oxohexyl) phthalate |
| MEP | Mono-ethyl phthalate |
| MiBP | Mono-isobutyl phthalate |
| MHBP | Mono-(3-hydroxybutyl) phthalate |
| MMP | Mono-methyl phthalate |
| MnOP | Mono-n-octyl phthalate |
| NOD | Non-obese diabetic |
| NOX2 | NADPH oxidase 2 |
| p53 | Tumor protein P53 |
| pCRH | Placental corticotropin-releasing hormone |
| PDX-1 | Pancreatic and duodenal homeobox 1 |
| PI3K/AKT signaling pathway | Phosphoinositide 3-kinase/Akt |
| PND | Postnatal day |
| PPAR | Peroxisome proliferator-activated receptors |
| PQQ | Pyrroloquinoline quinone |
| pSTAT1 | Phosphorylated signal transducer and activator of transcription 1 |
| ROS | Reactive oxygen species |
| SGLT2 | Sodium-glucose transport protein 2 |
| STZ | Streptozotocin |
| T1DM | Type 1 diabetes mellitus |
| T2DM | Type 2 diabetes mellitus |
| TNF-α | Tumor necrosis factor |
References
- Mariana, M.; Feiteiro, J.; Verde, I.; Cairrao, E. The effects of phthalates in the cardiovascular and reproductive systems: A review. Environ. Int. 2016, 94, 758–776. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.H.; Herianto, S.; Lee, C.C.; Hung, H.; Chen, H.L. The effects of phthalate ester exposure on human health: A review. Sci. Total Environ. 2021, 786, 147371. [Google Scholar] [CrossRef] [PubMed]
- Mariana, M.; Cairrao, E. Phthalates Implications in the Cardiovascular System. J. Cardiovasc. Dev. Dis. 2020, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, I.; Lorigo, M.; Cairrao, E. Update about the disrupting-effects of phthalates on the human reproductive system. Mol. Reprod. Dev. 2021, 88, 650–672. [Google Scholar] [CrossRef]
- Mariana, M.; Feiteiro, J.; Cairrao, E. Cardiovascular Response of Rat Aorta to Di-(2-ethylhexyl) Phthalate (DEHP) Exposure. Cardiovasc. Toxicol. 2018, 18, 356–364. [Google Scholar] [CrossRef]
- Zhang, H.; Ben, Y.; Han, Y.; Zhang, Y.; Li, Y.; Chen, X. Phthalate exposure and risk of diabetes mellitus: Implications from a systematic review and meta-analysis. Environ. Res. 2022, 204, 112109. [Google Scholar] [CrossRef]
- Shoshtari-Yeganeh, B.; Zarean, M.; Mansourian, M.; Riahi, R.; Poursafa, P.; Teiri, H.; Rafiei, N.; Dehdashti, B.; Kelishadi, R. Systematic review and meta-analysis on the association between phthalates exposure and insulin resistance. Environ. Sci. Pollut. Res. Int. 2019, 26, 9435–9442. [Google Scholar] [CrossRef]
- IDF. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021; Available online: https://diabetesatlas.org/atlas/tenth-edition/ (accessed on 24 August 2022).
- Latini, G.; De Felice, C.; Presta, G.; Del Vecchio, A.; Paris, I.; Ruggieri, F.; Mazzeo, P. In utero exposure to di-(2-ethylhexyl)phthalate and duration of human pregnancy. Environ. Health Perspect. 2003, 111, 1783–1785. [Google Scholar] [CrossRef] [Green Version]
- Silva, M.J.; Reidy, J.A.; Samandar, E.; Herbert, A.R.; Needham, L.L.; Calafat, A.M. Detection of phthalate metabolites in human saliva. Arch. Toxicol. 2005, 79, 647–652. [Google Scholar] [CrossRef]
- Main, K.M.; Mortensen, G.K.; Kaleva, M.M.; Boisen, K.A.; Damgaard, I.N.; Chellakooty, M.; Schmidt, I.M.; Suomi, A.M.; Virtanen, H.E.; Petersen, D.V.; et al. Human breast milk contamination with phthalates and alterations of endogenous reproductive hormones in infants three months of age. Environ. Health Perspect. 2006, 114, 270–276. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Park, M.J. Phthalate exposure and childhood obesity. Ann. Pediatr. Endocrinol. Metab. 2014, 19, 69–75. [Google Scholar] [CrossRef] [Green Version]
- Mathew, L.; Snyder, N.W.; Lyall, K.; Lee, B.K.; McClure, L.A.; Elliott, A.J.; Newschaffer, C.J. Prenatal phthalate exposure measurement: A comparison of metabolites quantified in prenatal maternal urine and newborn’s meconium. Sci. Total Environ. 2021, 796, 148898. [Google Scholar] [CrossRef]
- Brauner, E.V.; Uldbjerg, C.S.; Lim, Y.H.; Gregersen, L.S.; Krause, M.; Frederiksen, H.; Andersson, A.M. Presence of parabens, phenols and phthalates in paired maternal serum, urine and amniotic fluid. Environ. Int. 2022, 158, 106987. [Google Scholar] [CrossRef]
- Benjamin, S.; Masai, E.; Kamimura, N.; Takahashi, K.; Anderson, R.C.; Faisal, P.A. Phthalates impact human health: Epidemiological evidences and plausible mechanism of action. J. Hazard. Mater. 2017, 340, 360–383. [Google Scholar] [CrossRef]
- Hill, M.; Parizek, A.; Simjak, P.; Koucky, M.; Anderlova, K.; Krejci, H.; Vejrazkova, D.; Ondrejikova, L.; Cerny, A.; Kancheva, R. Steroids, steroid associated substances and gestational diabetes mellitus. Physiol. Res. 2021, 70, S617–S634. [Google Scholar] [CrossRef]
- Mirghani Dirar, A.; Doupis, J. Gestational diabetes from A to Z. World J. Diabetes 2017, 8, 489–511. [Google Scholar] [CrossRef]
- Filardi, T.; Panimolle, F.; Lenzi, A.; Morano, S. Bisphenol A and Phthalates in Diet: An Emerging Link with Pregnancy Complications. Nutrients 2020, 12, 525. [Google Scholar] [CrossRef] [Green Version]
- Bellavia, A.; Minguez-Alarcon, L.; Ford, J.B.; Keller, M.; Petrozza, J.; Williams, P.L.; Hauser, R.; James-Todd, T.; Team, E.S. Association of self-reported personal care product use with blood glucose levels measured during pregnancy among women from a fertility clinic. Sci. Total Environ. 2019, 695, 133855. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Jiao, Y.; Yan, H.; Liu, T.; Yan, H.; Yuan, J. Endocrine-disrupting chemicals and the risk of gestational diabetes mellitus: A systematic review and meta-analysis. Environ. Health 2022, 21, 53. [Google Scholar] [CrossRef] [PubMed]
- Predieri, B.; Bruzzi, P.; Bigi, E.; Ciancia, S.; Madeo, S.F.; Lucaccioni, L.; Iughetti, L. Endocrine Disrupting Chemicals and Type 1 Diabetes. Int. J. Mol. Sci. 2020, 21, 2937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howard, S.G. Exposure to environmental chemicals and type 1 diabetes: An update. J. Epidemiol. Community Health 2019, 73, 483–488. [Google Scholar] [CrossRef]
- Howard, S.G. Developmental Exposure to Endocrine Disrupting Chemicals and Type 1 Diabetes Mellitus. Front. Endocrinol. Lausanne 2018, 9, 513. [Google Scholar] [CrossRef] [PubMed]
- Del Chierico, F.; Rapini, N.; Deodati, A.; Matteoli, M.C.; Cianfarani, S.; Putignani, L. Pathophysiology of Type 1 Diabetes and Gut Microbiota Role. Int. J. Mol. Sci. 2022, 23, 14650. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, Y.; Tian, Y.; Huang, W.; Tong, N.; Fu, X. Integrative biology of extracellular vesicles in diabetes mellitus and diabetic complications. Theranostics 2022, 12, 1342–1372. [Google Scholar] [CrossRef] [PubMed]
- McKenney, R.L.; Short, D.K. Tipping the balance: The pathophysiology of obesity and type 2 diabetes mellitus. Surg. Clin. N. Am. 2011, 91, 1139–1148. [Google Scholar] [CrossRef]
- James-Todd, T.M.; Meeker, J.D.; Huang, T.; Hauser, R.; Ferguson, K.K.; Rich-Edwards, J.W.; McElrath, T.F.; Seely, E.W. Pregnancy urinary phthalate metabolite concentrations and gestational diabetes risk factors. Environ. Int. 2016, 96, 118–126. [Google Scholar] [CrossRef] [Green Version]
- James-Todd, T.; Ponzano, M.; Bellavia, A.; Williams, P.L.; Cantonwine, D.E.; Calafat, A.M.; Hauser, R.; Quinn, M.R.; Seely, E.W.; McElrath, T.F. Urinary phthalate and DINCH metabolite concentrations and gradations of maternal glucose intolerance. Environ. Int. 2022, 161, 107099. [Google Scholar] [CrossRef]
- Noor, N.; Ferguson, K.K.; Meeker, J.D.; Seely, E.W.; Hauser, R.; James-Todd, T.; McElrath, T.F. Pregnancy phthalate metabolite concentrations and infant birth weight by gradations of maternal glucose tolerance. Int. J. Hyg. Environ. Health 2019, 222, 395–401. [Google Scholar] [CrossRef]
- Shaffer, R.M.; Ferguson, K.K.; Sheppard, L.; James-Todd, T.; Butts, S.; Chandrasekaran, S.; Swan, S.H.; Barrett, E.S.; Nguyen, R.; Bush, N.; et al. Maternal urinary phthalate metabolites in relation to gestational diabetes and glucose intolerance during pregnancy. Environ. Int. 2019, 123, 588–596. [Google Scholar] [CrossRef]
- James-Todd, T.M.; Chiu, Y.H.; Messerlian, C.; Minguez-Alarcon, L.; Ford, J.B.; Keller, M.; Petrozza, J.; Williams, P.L.; Ye, X.; Calafat, A.M.; et al. Trimester-specific phthalate concentrations and glucose levels among women from a fertility clinic. Environ. Health 2018, 17, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, H.; Zhu, B.B.; Huang, K.; Zhu, Y.D.; Yan, S.Q.; Wu, X.Y.; Han, Y.; Sheng, J.; Cao, H.; Zhu, P.; et al. Effects of single and combined gestational phthalate exposure on blood pressure, blood glucose and gestational weight gain: A longitudinal analysis. Environ. Int. 2021, 155, 106677. [Google Scholar] [CrossRef]
- Liang, Q.X.; Lin, Y.; Fang, X.M.; Gao, Y.H.; Li, F. Association Between Phthalate Exposure in Pregnancy and Gestational Diabetes: A Chinese Cross-Sectional Study. Int. J. Gen. Med. 2022, 15, 179–189. [Google Scholar] [CrossRef]
- Chen, W.; He, C.; Liu, X.; An, S.; Wang, X.; Tao, L.; Zhang, H.; Tian, Y.; Wu, N.; Xu, P.; et al. Effects of exposure to phthalate during early pregnancy on gestational diabetes mellitus: A nested case-control study with propensity score matching. Environ. Sci. Pollut. Res. Int. 2022, 30, 33555–33566. [Google Scholar] [CrossRef]
- Wang, H.; Chen, R.; Gao, Y.; Qu, J.; Zhang, Y.; Jin, H.; Zhao, M.; Bai, X. Serum concentrations of phthalate metabolites in pregnant women and their association with gestational diabetes mellitus and blood glucose levels. Sci. Total Environ. 2023, 857, 159570. [Google Scholar] [CrossRef] [PubMed]
- Fisher, B.G.; Frederiksen, H.; Andersson, A.M.; Juul, A.; Thankamony, A.; Ong, K.K.; Dunger, D.B.; Hughes, I.A.; Acerini, C.L. Serum Phthalate and Triclosan Levels Have Opposing Associations With Risk Factors for Gestational Diabetes Mellitus. Front. Endocrinol. Lausanne 2018, 9, 99. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Ibarra, A.; Martinez-Razo, L.D.; Vazquez-Martinez, E.R.; Martinez-Cruz, N.; Flores-Ramirez, R.; Garcia-Gomez, E.; Lopez-Lopez, M.; Ortega-Gonzalez, C.; Camacho-Arroyo, I.; Cerbon, M. Unhealthy Levels of Phthalates and Bisphenol A in Mexican Pregnant Women with Gestational Diabetes and Its Association to Altered Expression of miRNAs Involved with Metabolic Disease. Int. J. Mol. Sci. 2019, 20, 3343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.; Just, A.C.; Colicino, E.; Calafat, A.M.; Oken, E.; Braun, J.M.; McRae, N.; Cantoral, A.; Pantic, I.; Pizano-Zarate, M.L.; et al. The associations of phthalate biomarkers during pregnancy with later glycemia and lipid profiles. Environ. Int. 2021, 155, 106612. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wu, M.; Gao, X.; Chen, J.; Li, S.; Chen, B.; Dong, R. Meconium Exposure to Phthalates, Sex and Thyroid Hormones, Birth Size and Pregnancy Outcomes in 251 Mother-Infant Pairs from Shanghai. Int. J. Environ. Res. Public Health 2020, 17, 7711. [Google Scholar] [CrossRef]
- Barrett, E.S.; Corsetti, M.; Day, D.; Thurston, S.W.; Loftus, C.T.; Karr, C.J.; Kannan, K.; LeWinn, K.Z.; Smith, A.K.; Smith, R.; et al. Prenatal phthalate exposure in relation to placental corticotropin releasing hormone (pCRH) in the CANDLE cohort. Environ. Int. 2022, 160, 107078. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, G.D.; Dodds, L.; Arbuckle, T.E.; Ashley-Martin, J.; Fraser, W.; Fisher, M.; Taback, S.; Keely, E.; Bouchard, M.F.; Monnier, P.; et al. Exposure to phthalates, bisphenol A and metals in pregnancy and the association with impaired glucose tolerance and gestational diabetes mellitus: The MIREC study. Environ. Int. 2015, 83, 63–71. [Google Scholar] [CrossRef] [Green Version]
- Robledo, C.A.; Peck, J.D.; Stoner, J.; Calafat, A.M.; Carabin, H.; Cowan, L.; Goodman, J.R. Urinary phthalate metabolite concentrations and blood glucose levels during pregnancy. Int. J. Hyg. Environ. Health 2015, 218, 324–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zukin, H.; Eskenazi, B.; Holland, N.; Harley, K.G. Prenatal exposure to phthalates and maternal metabolic outcomes in a high-risk pregnant Latina population. Environ. Res. 2021, 194, 110712. [Google Scholar] [CrossRef]
- Chen, M.; Zhao, S.; Guo, W.H.; Zhu, Y.P.; Pan, L.; Xie, Z.W.; Sun, W.L.; Jiang, J.T. Maternal exposure to Di-n-butyl phthalate (DBP) aggravate gestational diabetes mellitus via FoxM1 suppression by pSTAT1 signalling. Ecotoxicol. Environ. Saf. 2020, 205, 111154. [Google Scholar] [CrossRef]
- John, C.M.; Mohamed Yusof, N.I.S.; Abdul Aziz, S.H.; Mohd Fauzi, F. Maternal Cognitive Impairment Associated with Gestational Diabetes Mellitus-A Review of Potential Contributing Mechanisms. Int. J. Mol. Sci. 2018, 19, 3894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedman, J.E.; Kirwan, J.P.; Jing, M.; Presley, L.; Catalano, P.M. Increased skeletal muscle tumor necrosis factor-alpha and impaired insulin signaling persist in obese women with gestational diabetes mellitus 1 year postpartum. Diabetes 2008, 57, 606–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, T.; Wang, S.; Li, L.; Zhu, A.; Wang, Q. Associating diethylhexyl phthalate to gestational diabetes mellitus via adverse outcome pathways using a network-based approach. Sci. Total Environ. 2022, 824, 153932. [Google Scholar] [CrossRef]
- Sarath Josh, M.K.; Pradeep, S.; Vijayalekshmi Amma, K.S.; Balachandran, S.; Abdul Jaleel, U.C.; Doble, M.; Spener, F.; Benjamin, S. Phthalates efficiently bind to human peroxisome proliferator activated receptor and retinoid X receptor alpha, beta, gamma subtypes: An in silico approach. J. Appl. Toxicol. 2014, 34, 754–765. [Google Scholar] [CrossRef] [PubMed]
- Desvergne, B.; Feige, J.N.; Casals-Casas, C. PPAR-mediated activity of phthalates: A link to the obesity epidemic? Mol. Cell Endocrinol. 2009, 304, 43–48. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, H.Y.; Bae, S.; Lim, Y.H.; Hong, Y.C. Diethylhexyl phthalates is associated with insulin resistance via oxidative stress in the elderly: A panel study. PLoS ONE 2013, 8, e71392. [Google Scholar] [CrossRef] [Green Version]
- Cho, Y.J.; Park, S.B.; Han, M. Di-(2-ethylhexyl)-phthalate induces oxidative stress in human endometrial stromal cells in vitro. Mol. Cell Endocrinol. 2015, 407, 9–17. [Google Scholar] [CrossRef]
- Casals-Casas, C.; Desvergne, B. Endocrine disruptors: From endocrine to metabolic disruption. Annu. Rev. Physiol. 2011, 73, 135–162. [Google Scholar] [CrossRef] [Green Version]
- Bodin, J.; Stene, L.C.; Nygaard, U.C. Can exposure to environmental chemicals increase the risk of diabetes type 1 development? Biomed. Res. Int. 2015, 2015, 208947. [Google Scholar] [CrossRef]
- Castro-Correia, C.; Correia-Sa, L.; Norberto, S.; Delerue-Matos, C.; Domingues, V.; Costa-Santos, C.; Fontoura, M.; Calhau, C. Phthalates and type 1 diabetes: Is there any link? Environ. Sci. Pollut. Res. Int. 2018, 25, 17915–17919. [Google Scholar] [CrossRef] [Green Version]
- Bodin, J.; Kocbach Bolling, A.; Wendt, A.; Eliasson, L.; Becher, R.; Kuper, F.; Lovik, M.; Nygaard, U.C. Exposure to bisphenol A, but not phthalates, increases spontaneous diabetes type 1 development in NOD mice. Toxicol. Rep. 2015, 2, 99–110. [Google Scholar] [CrossRef] [Green Version]
- Weldingh, N.M.; Jorgensen-Kaur, L.; Becher, R.; Holme, J.A.; Bodin, J.; Nygaard, U.C.; Bolling, A.K. Bisphenol A Is More Potent than Phthalate Metabolites in Reducing Pancreatic beta-Cell Function. Biomed. Res. Int. 2017, 2017, 4614379. [Google Scholar] [CrossRef] [Green Version]
- Tiano, J.; Mauvais-Jarvis, F. Selective estrogen receptor modulation in pancreatic beta-cells and the prevention of type 2 diabetes. Islets 2012, 4, 173–176. [Google Scholar] [CrossRef] [Green Version]
- Engel, A.; Buhrke, T.; Imber, F.; Jessel, S.; Seidel, A.; Volkel, W.; Lampen, A. Agonistic and antagonistic effects of phthalates and their urinary metabolites on the steroid hormone receptors ERalpha, ERbeta, and AR. Toxicol. Lett. 2017, 277, 54–63. [Google Scholar] [CrossRef]
- Martinez-Arguelles, D.B.; Papadopoulos, V. Identification of hot spots of DNA methylation in the adult male adrenal in response to in utero exposure to the ubiquitous endocrine disruptor plasticizer di-(2-ethylhexyl) phthalate. Endocrinology 2015, 156, 124–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.; Sun, L.; Zhang, S.; Zhao, X.; Gang, X.; Wang, G. Evaluating the Causal Role of Gut Microbiota in Type 1 Diabetes and Its Possible Pathogenic Mechanisms. Front. Endocrinol. Lausanne 2020, 11, 125. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Raikhel, V.; Gopalakrishnan, K.; Fernandez-Hernandez, H.; Lambertini, L.; Manservisi, F.; Falcioni, L.; Bua, L.; Belpoggi, F.; Teitelbaum, S.L.; et al. Effect of postnatal low-dose exposure to environmental chemicals on the gut microbiome in a rodent model. Microbiome 2016, 4, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, C.; Kang, J.H.; Jeung, E.B. Calcium homeostasis in diabetes mellitus. J. Vet. Sci. 2017, 18, 261–266. [Google Scholar] [CrossRef]
- Johns, L.E.; Ferguson, K.K.; Meeker, J.D. Relationships Between Urinary Phthalate Metabolite and Bisphenol A Concentrations and Vitamin D Levels in U.S. Adults: National Health and Nutrition Examination Survey (NHANES), 2005–2010. J. Clin. Endocrinol. Metab. 2016, 101, 4062–4069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johns, L.E.; Ferguson, K.K.; Cantonwine, D.E.; McElrath, T.F.; Mukherjee, B.; Meeker, J.D. Urinary BPA and Phthalate Metabolite Concentrations and Plasma Vitamin D Levels in Pregnant Women: A Repeated Measures Analysis. Environ. Health Perspect. 2017, 125, 087026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batista-Silva, H.; Dambros, B.F.; Rodrigues, K.; Cesconetto, P.A.; Zamoner, A.; Sousa de Moura, K.R.; Gomes Castro, A.J.; Van Der Kraak, G.; Mena Barreto Silva, F.R. Acute exposure to bis(2-ethylhexyl)phthalate disrupts calcium homeostasis, energy metabolism and induces oxidative stress in the testis of Danio rerio. Biochimie 2020, 175, 23–33. [Google Scholar] [CrossRef]
- Liu, P.S.; Chen, Y.Y. Butyl benzyl phthalate blocks Ca2+ signaling coupled with purinoceptor in rat PC12 cells. Toxicol. Appl. Pharmacol. 2006, 210, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, R.; Teshima, R.; Sawada, J. Effect of dialkyl phthalates on the degranulation and Ca2+ response of RBL-2H3 mast cells. Immunol. Lett. 2002, 80, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Posnack, N.G.; Idrees, R.; Ding, H.; Jaimes, R., 3rd; Stybayeva, G.; Karabekian, Z.; Laflamme, M.A.; Sarvazyan, N. Exposure to phthalates affects calcium handling and intercellular connectivity of human stem cell-derived cardiomyocytes. PLoS ONE 2015, 10, e0121927. [Google Scholar] [CrossRef]
- Sol, C.M.; Santos, S.; Duijts, L.; Asimakopoulos, A.G.; Martinez-Moral, M.P.; Kannan, K.; Jaddoe, V.W.V.; Trasande, L. Fetal phthalates and bisphenols and childhood lipid and glucose metabolism. A population-based prospective cohort study. Environ. Int. 2020, 144, 106063. [Google Scholar] [CrossRef]
- Attina, T.M.; Trasande, L. Association of Exposure to Di-2-Ethylhexylphthalate Replacements With Increased Insulin Resistance in Adolescents From NHANES 2009–2012. J. Clin. Endocrinol. Metab. 2015, 100, 2640–2650. [Google Scholar] [CrossRef] [Green Version]
- Carlsson, A.; Sorensen, K.; Andersson, A.M.; Frederiksen, H.; Juul, A. Bisphenol A, phthalate metabolites and glucose homeostasis in healthy normal-weight children. Endocr. Connect. 2018, 7, 232–238. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.Y.; Hwang, J.S.; Sung, F.C.; Lin, C.Y.; Hsieh, C.J.; Chen, P.C.; Su, T.C. Mono-2-ethylhexyl phthalate associated with insulin resistance and lower testosterone levels in a young population. Environ. Pollut. 2017, 225, 112–117. [Google Scholar] [CrossRef]
- Dales, R.E.; Kauri, L.M.; Cakmak, S. The associations between phthalate exposure and insulin resistance, beta-cell function and blood glucose control in a population-based sample. Sci. Total Environ. 2018, 612, 1287–1292. [Google Scholar] [CrossRef]
- Dirinck, E.; Dirtu, A.C.; Geens, T.; Covaci, A.; Van Gaal, L.; Jorens, P.G. Urinary phthalate metabolites are associated with insulin resistance in obese subjects. Environ. Res. 2015, 137, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Nam, D.J.; Kim, Y.; Yang, E.H.; Lee, H.C.; Ryoo, J.H. Relationship between urinary phthalate metabolites and diabetes: Korean National Environmental Health Survey (KoNEHS) cycle 3 (2015–2017). Ann. Occup. Environ. Med. 2020, 32, e34. [Google Scholar] [CrossRef]
- Duan, Y.; Sun, H.; Han, L.; Chen, L. Association between phthalate exposure and glycosylated hemoglobin, fasting glucose, and type 2 diabetes mellitus: A case-control study in China. Sci. Total Environ. 2019, 670, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Zhao, S.; Zhang, H.; Chen, J.; Zhang, M.; Wang, M.; Wu, M.; Li, S.; Chen, B. Sex Differences in the Association of Urinary Concentrations of Phthalates Metabolites with Self-Reported Diabetes and Cardiovascular Diseases in Shanghai Adults. Int. J. Environ. Res. Public Health 2017, 14, 598. [Google Scholar] [CrossRef] [Green Version]
- Al-Bazi, M.M.; Kumosani, T.A.; Al-Malki, A.L.; Kurunthachalam, K.; Moselhy, S.S. Screening the incidence of diabetogensis with urinary phthalate in Saudi subjects. Environ. Sci. Pollut. Res. Int. 2022, 29, 28743–28748. [Google Scholar] [CrossRef] [PubMed]
- Bai, P.Y.; Wittert, G.; Taylor, A.W.; Martin, S.A.; Milne, R.W.; Jenkins, A.J.; Januszewski, A.S.; Shi, Z. The association between total phthalate concentration and non-communicable diseases and chronic inflammation in South Australian urban dwelling men. Environ. Res. 2017, 158, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Svensson, K.; Hernandez-Ramirez, R.U.; Burguete-Garcia, A.; Cebrian, M.E.; Calafat, A.M.; Needham, L.L.; Claudio, L.; Lopez-Carrillo, L. Phthalate exposure associated with self-reported diabetes among Mexican women. Environ. Res. 2011, 111, 792–796. [Google Scholar] [CrossRef] [Green Version]
- James-Todd, T.; Stahlhut, R.; Meeker, J.D.; Powell, S.G.; Hauser, R.; Huang, T.; Rich-Edwards, J. Urinary phthalate metabolite concentrations and diabetes among women in the National Health and Nutrition Examination Survey (NHANES) 2001–2008. Environ. Health Perspect. 2012, 120, 1307–1313. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Cornelis, M.C.; Townsend, M.K.; Tobias, D.K.; Eliassen, A.H.; Franke, A.A.; Hauser, R.; Hu, F.B. Association of urinary concentrations of bisphenol A and phthalate metabolites with risk of type 2 diabetes: A prospective investigation in the Nurses′ Health Study (NHS) and NHSII cohorts. Environ. Health Perspect. 2014, 122, 616–623. [Google Scholar] [CrossRef] [Green Version]
- Duan, Y.; Sun, H.; Yao, Y.; Han, L.; Chen, L. Perturbation of serum metabolome in relation to type 2 diabetes mellitus and urinary levels of phthalate metabolites and bisphenols. Environ. Int. 2021, 155, 106609. [Google Scholar] [CrossRef]
- Mengozzi, A.; Carli, F.; Guiducci, L.; Parolini, F.; Biancalana, E.; Gastaldelli, A.; Solini, A. SGLT2 inhibitors and thiazide enhance excretion of DEHP toxic metabolites in subjects with type 2 diabetes: A randomized clinical trial. Environ. Res. 2021, 192, 110316. [Google Scholar] [CrossRef]
- Rajesh, P.; Balasubramanian, K. Gestational exposure to di(2-ethylhexyl) phthalate (DEHP) impairs pancreatic beta-cell function in F1 rat offspring. Toxicol. Lett. 2015, 232, 46–57. [Google Scholar] [CrossRef]
- Rajagopal, G.; Bhaskaran, R.S.; Karundevi, B. Maternal di-(2-ethylhexyl) phthalate exposure alters hepatic insulin signal transduction and glucoregulatory events in rat F(1) male offspring. J. Appl. Toxicol. 2019, 39, 751–763. [Google Scholar] [CrossRef]
- Rajagopal, G.; Bhaskaran, R.S.; Karundevi, B. Developmental exposure to DEHP alters hepatic glucose uptake and transcriptional regulation of GLUT2 in rat male offspring. Toxicology 2019, 413, 56–64. [Google Scholar] [CrossRef]
- Deng, T.; Zhang, Y.; Wu, Y.; Ma, P.; Duan, J.; Qin, W.; Yang, X.; Chen, M. Dibutyl phthalate exposure aggravates type 2 diabetes by disrupting the insulin-mediated PI3K/AKT signaling pathway. Toxicol. Lett. 2018, 290, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Xu, T.; Mao, G.; Chen, Y.; Qiu, X.; Yang, L.; Zhao, T.; Xu, X.; Feng, W.; Wu, X. Di-(2-ethylhexyl) phthalate-induced hepatotoxicity exacerbated type 2 diabetes mellitus (T2DM) in female pubertal T2DM mice. Food Chem. Toxicol. 2021, 149, 112003. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Gao, K.; Liu, Y.; Mao, G.; Chen, K.; Qiu, X.; Zhao, T.; Yang, L.; Feng, W.; Wu, X. Transcriptome analysis revealed the mechanism of the metabolic toxicity and susceptibility of di-(2-ethylhexyl)phthalate on adolescent male ICR mice with type 2 diabetes mellitus. Arch. Toxicol. 2019, 93, 3183–3206. [Google Scholar] [CrossRef]
- Karabulut, G.; Barlas, N. The possible effects of mono butyl phthalate (MBP) and mono (2-ethylhexyl) phthalate (MEHP) on INS-1 pancreatic beta cells. Toxicol. Res. Camb 2021, 10, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Guven, C.; Dal, F.; Aydogan Ahbab, M.; Taskin, E.; Ahbab, S.; Adin Cinar, S.; Sirma Ekmekci, S.; Gulec, C.; Abaci, N.; Akcakaya, H. Low dose monoethyl phthalate (MEP) exposure triggers proliferation by activating PDX-1 at 1.1B4 human pancreatic beta cells. Food Chem. Toxicol. 2016, 93, 41–50. [Google Scholar] [CrossRef]
- Al-Abdulla, R.; Ferrero, H.; Soriano, S.; Boronat-Belda, T.; Alonso-Magdalena, P. Screening of Relevant Metabolism-Disrupting Chemicals on Pancreatic beta-Cells: Evaluation of Murine and Human In Vitro Models. Int. J. Mol. Sci. 2022, 23, 4182. [Google Scholar] [CrossRef]
- Mondal, S.; Mukherjee, S. Long-term dietary administration of diethyl phthalate triggers loss of insulin sensitivity in two key insulin target tissues of mice. Hum. Exp. Toxicol. 2020, 39, 984–993. [Google Scholar] [CrossRef]
- Schaffert, A.; Karkossa, I.; Ueberham, E.; Schlichting, R.; Walter, K.; Arnold, J.; Bluher, M.; Heiker, J.T.; Lehmann, J.; Wabitsch, M.; et al. Di-(2-ethylhexyl) phthalate substitutes accelerate human adipogenesis through PPARgamma activation and cause oxidative stress and impaired metabolic homeostasis in mature adipocytes. Environ. Int. 2022, 164, 107279. [Google Scholar] [CrossRef] [PubMed]
- She, Y.; Jiang, L.; Zheng, L.; Zuo, H.; Chen, M.; Sun, X.; Li, Q.; Geng, C.; Yang, G.; Jiang, L.; et al. The role of oxidative stress in DNA damage in pancreatic beta cells induced by di-(2-ethylhexyl) phthalate. Chem. Biol. Interact. 2017, 265, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Zheng, J.; Qin, J.; Liu, S.; Liu, X.; Gu, Y.; Yang, S.; Du, J.; Li, S.; Chen, B.; et al. Dibutyl phthalate affects insulin synthesis and secretion by regulating the mitochondrial apoptotic pathway and oxidative stress in rat insulinoma cells. Ecotoxicol. Environ. Saf. 2023, 249, 114396. [Google Scholar] [CrossRef]
- Viswanathan, M.P.; Mullainadhan, V.; Chinnaiyan, M.; Karundevi, B. Effects of DEHP and its metabolite MEHP on insulin signalling and proteins involved in GLUT4 translocation in cultured L6 myotubes. Toxicology 2017, 386, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Shen, X.Y.; Zhang, W.W.; Chen, H.; Xu, W.P.; Wei, W. Di-(2-ethylhexyl) phthalate could disrupt the insulin signaling pathway in liver of SD rats and L02 cells via PPARgamma. Toxicol. Appl. Pharmacol. 2017, 316, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Baralic, K.; Zivancevic, K.; Jorgovanovic, D.; Javorac, D.; Radovanovic, J.; Gojkovic, T.; Buha Djordjevic, A.; Curcic, M.; Mandinic, Z.; Bulat, Z.; et al. Probiotic reduced the impact of phthalates and bisphenol A mixture on type 2 diabetes mellitus development: Merging bioinformatics with in vivo analysis. Food Chem. Toxicol. 2021, 154, 112325. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Kamijima, M.; Nakajima, T. Di(2-ethylhexyl) phthalate-induced toxicity and peroxisome proliferator-activated receptor alpha: A review. Environ. Health Prev. Med. 2019, 24, 47. [Google Scholar] [CrossRef] [Green Version]
- Duan, Y.; Wang, L.; Han, L.; Wang, B.; Sun, H.; Chen, L.; Zhu, L.; Luo, Y. Exposure to phthalates in patients with diabetes and its association with oxidative stress, adiponectin, and inflammatory cytokines. Environ. Int. 2017, 109, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Li, A.J.; Martinez-Moral, M.P.; Al-Malki, A.L.; Al-Ghamdi, M.A.; Al-Bazi, M.M.; Kumosani, T.A.; Kannan, K. Mediation analysis for the relationship between urinary phthalate metabolites and type 2 diabetes via oxidative stress in a population in Jeddah, Saudi Arabia. Environ. Int. 2019, 126, 153–161. [Google Scholar] [CrossRef]
- Dong, R.; Chen, J.; Zheng, J.; Zhang, M.; Zhang, H.; Wu, M.; Li, S.; Chen, B. The role of oxidative stress in cardiometabolic risk related to phthalate exposure in elderly diabetic patients from Shanghai. Environ. Int. 2018, 121, 340–348. [Google Scholar] [CrossRef]
- Stojanoska, M.M.; Milosevic, N.; Milic, N.; Abenavoli, L. The influence of phthalates and bisphenol A on the obesity development and glucose metabolism disorders. Endocrine 2017, 55, 666–681. [Google Scholar] [CrossRef]
- Song, J.W.; Chung, K.C. Observational studies: Cohort and case-control studies. Plast. Reconstr. Surg. 2010, 126, 2234–2242. [Google Scholar] [CrossRef] [Green Version]
- Wiberg, B.; Lind, P.M.; Lind, L. Serum levels of monobenzylphthalate (MBzP) is related to carotid atherosclerosis in the elderly. Environ. Res. 2014, 133, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Trasande, L.; Lampa, E.; Lind, L.; Lind, P.M. Population attributable risks and costs of diabetogenic chemical exposures in the elderly. J. Epidemiol. Community Health 2017, 71, 111–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Study Type | Phthalate | Biological Sample | Population | Findings | Ref | |||
|---|---|---|---|---|---|---|---|---|
| Matrix | Quantity | Country | Size | Age | ||||
| Cohort | MEP, MBP, MCPP, ∑DEHP | Urine Blood | 4 Gestational weeks 10, 18, 26, 35 | USA | 350 | 31.9 (mean) |
| [27] |
| Cohort | MiBP, MHBP MBP, MCNP, MCPP | Urine | 4 Gestational weeks 10, 18, 26, 35 | USA | 606 | 33.5 (mean) |
| [28] |
| Cohort | - | Urine | 4 Gestational weeks 10, 18, 26, 35 | USA | 350 | 31.9 (mean) | No association | [29] |
| Cohort | MEP, MBP, MCOP, MCPP | Urine | 2 1st and 3rd trimesters | USA | 705 | 31 (mean) |
| [30] |
| Cohort | MEP, MiBP | Urine | 3 Each trimester | USA | 245 | 35.3 (mean) |
| [31] |
| Cohort | Phthalates | Blood | 1 Late 2nd trimester | USA | 233 | 35.4 (mean) |
| [19] |
| Cohort | MBP, MMP, MEOHP, MEHHP | Urine Serum | 3 Each trimester | China | 3269 | 24–35 |
| [32] |
| Case-control | MEHP, MMP, MEP, MiBP, MECPP, MEOHP | Urine Blood | 1 Early 3rd trimester | China | 200 | 32 (mean) |
| [33] |
| Case-control | MnOP, MBzP, MEOHP, MECPP | Urine | 1 1st trimester | China | 676 | 20–35 |
| [34] |
| Case-control | MBP, MiBP | Serum | 1 Childbirth | China | 201 | 22–43 |
| [35] |
| Case-control | MiBP, MEHP, MCOP | Serum | 1 Gestational weeks 10–17 | UK | 232 | ≈33 (mean) |
| [36] |
| Case-control | MBzP, MBP, MEHP, MiBP | Urine Blood | 1 2nd trimester | Mexico | 40 | 24–45 |
| [37] |
| Cohort | MECPTP, ∑DBP | Urine Blood | 2 | Mexico | 618 | 27.3 (mean) |
| [38] |
| ||||||||
| Cross-sectional | MBP, MiBP, MEHP | Meconium | 1 Childbirth | China | 251 | 29 (mean) |
| [39] |
| Cohort | Phthalate mixtures | Urine Blood | 2 Late 2nd and 3rd trimesters | USA | 1018 | 26.4 (mean) |
| [40] |
| Cohort | - | Urine | 1 1st trimester | Canada | 1274 | ≥18 | No association | [41] |
| Cohort | - | Urine | 1 1st or 2nd trimesters | USA | 72 | 22 (mean) | No association | [42] |
| Cohort | - | Urine | 2 Late 1st and 2nd trimesters | USA | 415 | 18–45 | No association | [43] |
| Study Type | Phthalate | Biological Sample | Population | Findings | Ref | |||
|---|---|---|---|---|---|---|---|---|
| Country | Size | Age | Gender | |||||
| Cohort | HMWP LMWP |
| Netherlands | 757 | Mother: 31 Child: 9.7 (mean) | M/F | Sex specific effects for boys: | [69] |
| ||||||||
| Cross-sectional | DEHP, DINP |
| USA | 356 | 12–19 | M/F |
| [70] |
| Cross-sectional | - |
| Denmark | 107 | 12 (mean) | M/F | No association | [71] |
| Cross-sectional | MEHP |
| Taiwan | 786 | 12–30 | M/F | In young adults (20–30 years old) group: | [72] |
| ||||||||
| Cross-sectional | MBzP, MiBP, MCPP, MEHP, MEHHP, ∑DEHP |
| Canada | 2119 | 12–79 | M/F |
| [73] |
| Cross-sectional | Phthalate metabolites |
| Belgium | 123 | 18–84 | M/F |
| [74] |
| Cross-sectional | MBzP, MBP, MCPP, DEHP, MEOHP, MEHHP |
| South Korea | 3781 | 19- ≥ 70 | M/F |
| [75] |
| Case-control | MEHHP, MEOHP, MEHP, MCPP, MiBP, MMP, ∑DEHP, MECPP, MCMHP |
| China | 500 | Case: 58 Control: 51 (mean) | M/F |
| [76] |
| Cross-sectional | MEOHP, MEHHP, MECPP |
| China | 2330 | 53 (mean) | M/F | Sex specific effects for men: | [77] |
| ||||||||
| Case-control | MEP, MEOHP, MBP |
| Saudi Arabia | 150 | 45 (mean) | M |
| [78] |
| Cross-sectional | Total phthalates |
| Australia | 1504 | 39–84 | M |
| [79] |
| Case-control | MBzP, MEOHP, MEHHP, MECPP |
| Mexico | 221 | Case: 60.5 Control: 52.4 (mean) | F |
| [80] |
| Cross-sectional | MBP, MBzP, MiBP, MCPP, ∑DEHP |
| USA | 2350 | 20–79 | F |
| [81] |
| Case-control | ∑DEHP, ∑DBP |
| USA | 1941 | Premenopausal: 45.6 Postmenopausal: 65.6 (mean) | F |
| [82] |
| Cohort | MECPTP, DBP |
| Mexico | 618 | 27.7 (mean) | F |
| [38] |
| Case-control | ∑DEHP, MCPP, MiBP, MMP |
| China | 120 | 56 (mean) | M/F |
| [83] |
| Clinical trial | DEHP metabolites |
| Italy | 30 | 60 (mean) | M/F |
| [84] |
| Study Type | Animal/Cell Type | Phthalate | Treatment | Findings | Ref | |
|---|---|---|---|---|---|---|
| Dose/Concentration | Duration | |||||
| In Vivo | Pregnant Wistar rats | DEHP | 1, 10, 100 mg/Kg/day | GD 9 to GD 21 | Changes in expression of insulin gene transcription and glucose sensing mechanism-related genes leading to β-cell dysfunction in F1 offspring. | [85] |
| In Vivo | Pregnant Wistar rats | DEHP | 10, 100 mg/Kg/day | GD 9 to PND 21 | Impaired insulin signal transduction and glucoregulatory events in F1 male offspring leading to decreased glucose tolerance, IR, and hyperglycemia. | [86] |
| In Vivo | Pregnant Wistar rats | DEHP | 10, 100 mg/Kg/day | GD 9 to PND 21 | Impaired regulation of GLUT2 gene and epigenetic changes in IR and GLUT2 gene promoters. | [87] |
| In Vivo | Male Balb/c mice (5–6 weeks old) | DBP | 0.5, 5, 50 mg/Kg/day | 7 weeks | Highest DBP dose decreased insulin secretion and glucose intolerance. T2DM mouse model: IR, organ lesions, decreased PI3K/AKT signaling pathway, increased pancreatic GLUT2. Administration of selective insulin receptor activator (DMAQ-B1) decreased the adverse effects on insulin deficiency and resistance. | [88] |
| In Vivo | Female ICR mice with and w/o T2DM (3 weeks old) | DEHP | 0.18, 1.8, 18, 180 mg/Kg/day | 3 weeks | Female T2DM mice more susceptible to DEHP than male and normal mice. Activation of JNK and impaired insulin sensitivity in the liver. | [89] |
| In Vivo | Male ICR mice with and w/o T2DM (3 weeks old) | DEHP | 0.18, 1.8, 18, 180 mg/Kg/day | 3 weeks | Impaired endocrine and metabolic functions. Increased IR. T2DM mice more susceptible to DEHP than normal mice. | [90] |
| In Vitro | Rat pancreatic β-cell line (INS-1) | MEHP, MBP | 0.001–10 µM | 24, 48, 72 h | Decreased cell viability. Increased oxidative stress. Gene expression changes related to pancreatic β-cell function and apoptosis. | [91] |
| In Vitro | Rat pancreatic β-cell line (INS-1E) | MEHP, MBP, MiBP | 5, 50, 500 µM | 2, 24, 48, 72 h | Decreased potency of phthalates, compared to BPA, affected insulin secretion. | [56] |
| In Vitro | Human pancreatic β-cells (1.1B4) | MEP | 1–1000 nM | 24 h | Increased insulin secretion, possibly involving ERα, PPARγ, and PDX-1. | [92] |
| In Vitro | Murine pancreatic β-cell line (MIN6) Human pancreatic β-cell line (EndoC-βH1) | DEHP | 100 pM-10 µM | 24, 48, 72 h, or 7 days | Impaired insulin secretion in both cell lines. | [93] |
| In Vivo | Male Swiss albino mice (8-week-old) | DEP | 1, 10 mg/Kg.bw/day | 3 months | Chronic exposure leading to impaired insulin signaling in hepatocytes and adipocytes. Increased NOX2 levels involved in the generation of ROS. | [94] |
| In Vitro | Differentiated human preadipocytes of the Simpson-Golabi-Behmel syndrome (SGBS) cell line | DINP, DPHP | 0.01–100 µM | Preadipocytes: 16 days Mature adipocytes: 8 days | Activation of PPARγ in preadipocytes leading to lipid accumulation and adipogenesis. Lipid storage, oxidative stress, and impaired adipokine release in mature adipocytes. | [95] |
| In Vitro | Rat pancreatic β-cell line (INS-1) | DEHP | MTT: 0–1600 µM Other experiments: 0–400 µM | 1 h or 24 h | Involvement of the lysosome–mitochondrial axis pathway through oxidative stress and p53 and ATM activation. Protective effect of PQQ. | [96] |
| In Vitro | Rat pancreatic β-cell line (INS-1) | DBP | MTT: 15, 30, 60, 120 µM Other experiments: 15, 30, 60 µM | 24 h | Altered PDX-1 and GLUT-2 levels, which reduced insulin synthesis and secretion through mitochondrial apoptotic pathway and oxidative stress. | [97] |
| In Vitro | Rat skeletal muscle model (L6 myoblast cells) | DEHP, MEHP | 50, 100 µM | 24 h | Changes in GLUT4 levels and translocation. Changes in insulin signaling molecules. | [98] |
| In Vivo In Vitro | Male Sprague Dawley rats Human hepatocyte cell line (L02) | DEHP | In Vivo: 0.05, 5, 500 mg/Kg.bw In Vitro: 5, 10, 25, 50, 100 µM | In Vivo: 15 weeks In Vitro: 24, 48 h | In Vivo: liver damage, glucose and insulin tolerance, reduced insulin receptor and GLUT4 protein expression. In Vitro: interaction with PPARγ, increased ROS levels, reduced insulin receptor and GLUT4 protein expression. | [99] |
| In Silico In Vivo | Male albino rats | DEHP, DBP, (BPA) | 50 mg/Kg.bw/day (25 mg/Kg.bw/day) | 28 days | Joint action of DEHP, DBP, and BPA led to T2DM through oxidative stress and apoptosis. Protective role of probiotic mixture regarding redox properties in the pancreas. | [100] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mariana, M.; Cairrao, E. The Relationship between Phthalates and Diabetes: A Review. Metabolites 2023, 13, 746. https://doi.org/10.3390/metabo13060746
Mariana M, Cairrao E. The Relationship between Phthalates and Diabetes: A Review. Metabolites. 2023; 13(6):746. https://doi.org/10.3390/metabo13060746
Chicago/Turabian StyleMariana, Melissa, and Elisa Cairrao. 2023. "The Relationship between Phthalates and Diabetes: A Review" Metabolites 13, no. 6: 746. https://doi.org/10.3390/metabo13060746
APA StyleMariana, M., & Cairrao, E. (2023). The Relationship between Phthalates and Diabetes: A Review. Metabolites, 13(6), 746. https://doi.org/10.3390/metabo13060746