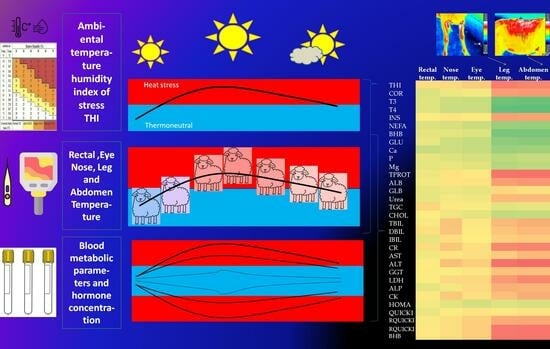
Influence of Heat Stress on Body Temperatures Measured by Infrared Thermography, Blood Metabolic Parameters and Its Correlation in Sheep
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Management
2.2. Body Temperature and Ambient Temperature–Humidity Index
2.3. Blood Sampling and Metabolic Parameters Analysis
2.4. Statistical Analysis
3. Results
3.1. Temperature–Humidity Index (THI) and Environmental Stress
3.2. Body Temperature Measurement
3.3. Blood Metabolic Parameters
3.4. Correlation between Blood Parameters and Body Temperature
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berihulay, H.; Abied, A.; He, X.; Jiang, L.; Ma, Y. Adaptation Mechanisms of Small Ruminants to Environmental Heat Stress. Animals 2019, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Li, F.K.; Yang, Y.; Jenna, K.; Xia, C.H.; Lv, S.J.; Wei, W.H. Effect of Heat Stress on the Behavioral and Physiological Patterns of Small-Tail Han Sheep Housed Indoors. Trop. Anim. Health Prod. 2018, 50, 1893–1901. [Google Scholar] [CrossRef] [PubMed]
- Henry, B.K.; Eckard, R.J.; Beauchemin, K.A. Review: Adaptation of Ruminant Livestock Production Systems to Climate Changes. Animal 2018, 12, S445–S456. [Google Scholar] [CrossRef]
- Kochewad, S.A.; Traghunandan, K.R.; Reddy, K.K.; Kumari, N.; Ramana, D.; Kumar, S.; Meena., L.R.; Singh, S.P. Reproductive Performance and Body Condition Score of Deccani Sheep during Various Physiological Stages in Different Farming Systems. Indian J. Anim. Sci. 2018, 88, 1379–1382. [Google Scholar] [CrossRef]
- Kochewad, S.A.; Raghunandan, T.; Sarjan Rao, K.; Kondal Reddy, K.; Nalini Kumari, N.; Ramana, D.B.V.; Singh, M. Productive Performance, Body Condition Score and Carcass Characteristics of Deccani Lambs Reared under Different Farming Systems. Indian J. Anim. Res 2018, 52, 444–448. [Google Scholar] [CrossRef]
- Shekhawat, I.; Pareek, A. A Review: Growth and Physiological Adaptability of Sheep to Heat Stress under Semi-Arid Environment. Int. J. Emerg. Trends Sci. Technol. 2015, 02, 3188–3198. [Google Scholar] [CrossRef]
- Lallo, C.; Cohen, J.; Rankine, D.; Taylor, M.; Cambell, J.; Stephenson, T. Characterizing heat stress on livestock using the temperature humidity index (thi)—Prospects for a warmer caribbean. Reg. Environ. Change 2018, 18, 2329–2340. [Google Scholar] [CrossRef]
- Lewis Baida, B.E.; Swinbourne, A.M.; Barwick, J.; Leu, S.T.; van Wettere, W.H.E.J. Technologies for the Automated Collection of Heat Stress Data in Sheep. Anim. Biotelemetry 2021, 9, 4. [Google Scholar] [CrossRef]
- Rashid, M.M.; Hossain, M.M.; Azad, M.A.K.; Hashem, M.A. Long term cyclic heat stress influences physiological responses and blood characteristics in indigenous sheep. BJAS 2013, 42, 96–100. [Google Scholar] [CrossRef]
- Knížková, İ.; Kunc, P.; Alp, G.; Gürdil, K.; Kemal, Y.P.; Omu, C.S. Applications of infrared thermography in animal production. J. Fac. Agric. 2007, 22, 329–336. [Google Scholar]
- McManus, C.; Tanure, C.B.; Peripolli, V.; Seixas, L.; Fischer, V.; Gabbi, A.M.; Costa, J.B.G., Jr. Infrared thermography in animal production: An overview. Comput Electron. Agric. 2016, 123, 10–16. [Google Scholar] [CrossRef]
- Soerensen, D.D.; Pedersen, L.J. Infrared skin temperature measurements for monitoring health in pigs: A review. Acta Vet. Scand. 2015, 57, 1–11. [Google Scholar] [CrossRef]
- Unruh, E.M.; Theurer, M.E.; White, B.J.; Larson, R.L.; Drouillard, J.S.; Schrag, N. Evaluation of infrared thermography as a diagnostic tool to predict heat stress events in feedlot cattle. Am. J. Vet. Res. 2017, 78, 771–777. [Google Scholar] [CrossRef] [PubMed]
- McManus, C.; Bianchini, E.; Do, T.; Paim, P.; Gontijo De Lima, F.; Neto, J.B.; Castanheira, M.; Ferreira Esteves, G.I.; Cardoso, C.C.; Dalcin, V.C. Infrared Thermography to Evaluate Heat Tolerance in Different Genetic Groups of Lambs. Sensors 2015, 15, 17258–17273. [Google Scholar] [CrossRef]
- Menchetti, L.; Nanni Costa, L.; Zappaterra, M.; Padalino, B. Effects of Reduced Space Allowance and Heat Stress on Behavior and Eye Temperature in Unweaned Lambs: A Pilot Study. Animals 2021, 11, 3464. [Google Scholar] [CrossRef] [PubMed]
- Stelletta, C.; Vencato, J.; Fiore, E.; Gianesella, M. Infrared thermography in reproduction. In Thermography Current Status and Advances in Livestock Animals and in Veterinary Medicine; Fondazione Iniziative Zooprofilattiche e Zootecniche: Brescia, Italy, 2013; pp. 113–125. [Google Scholar]
- Marai, I.F.M.; El- Darawany, A.A.; Fadiel, A.; Abdel-Hafez, M.A.M. Reproductive performance traits as affected by heat stress and its alleviation in sheep. Trop. Subtrop. Agroecosystems 2008, 8, 209–234. [Google Scholar]
- Marai, I.F.M.; El-Darawany, A.A.; Fadiel, A.; Abdel-Hafez, M.A.M. Physiological traits as affected by heat stress in sheep—A review. Small Rumin. Res. 2007, 71, 1–12. [Google Scholar] [CrossRef]
- Sejian, V.; Maurya, V.P.; Naqvi, S.M. Adaptive capability as indicated by endocrine and biochemical responses of Malpura ewes subjected to combined stresses (thermal and nutritional) in a semi-arid tropical environment. Int. J. Biometeorol. 2010, 54, 653–661. [Google Scholar] [CrossRef]
- Macias-Cruz, U.; Alvarez-Valenzuela, F.D.; Correa-Calderon, A.; Diaz-Molina, R.; Mellado, M.; Meza-Herrera, C.A. Thermoregulation of nutrient-restricted hair ewes subjected to heat stress during late pregnancy. J. Therm. Biol. 2013, 38, 1–9. [Google Scholar] [CrossRef]
- Sejian, V.; Bhatta, R.; Gaughan, J.; Malik, P.K.; Naqvi, S.M.K.; Lal, R. Adapting sheep production to climate change. In Sheep Production Adapting to Climate Change, 1st ed.; Springer: Singapore, 2017; pp. 1–29. [Google Scholar] [CrossRef]
- Pérez, R.V.; Macías Cruz, U.; Avendaño Reyes, L.; Correa-Calderón, A.; López Baca, M.D.L.Á.; Lara Rivera, A.L. Heat Stress Impacts in Hair Sheep Production. Review. Rev. Mex. Cienc. Pecu. 2020, 11, 205–222. [Google Scholar] [CrossRef]
- Hristov, S.; Joksimovic-Todorovic, M.; Milanov, S.; Trenic, V. Minimum standards of sheep and goat housing conditions and welfare. In Proceedings of the International Conference on Animal Welfare and Biosecurity on Farms in Serbia, Zemun, Serbia, 14–15 November 2007; pp. 149–157. [Google Scholar]
- Guyot, H.; Detilleux, J.; Lebreton, P.; Garnier, C.; Bonvoisin, M.; Rollin, F.; Sandersen, C. Comparison of Various Indices of Energy Metabolism in Recumbent and Healthy Dairy Cows. PLoS ONE 2017, 12, e0169716. [Google Scholar] [CrossRef] [PubMed]
- Hossein-Zadeh, N.G. Effects of main reproductive and health problems on the performance of dairy cows: A review. Span. J. Agric. Res. 2013, 3, 718–735. [Google Scholar] [CrossRef]
- Broucek, J.; Ryba, S.; Mihina, S.; Uhrincat, M.; Kisac, P. Impact of thermal-humidity index on milk yield under conditions of different dairy management. J. Anim. Feed Sci. 2007, 16, 329. [Google Scholar] [CrossRef]
- Könyves, T.; Zlatkovic, N.; Memisi, N.; Lukac, D.; Puvaca, N.; Stojsin, M.; Miscevic, B. Relationship of temperature-humidity index with milk production and feed intake of holstein-frisian cows in different year seasons. Thai J. Vet. Med. 2017, 47, 15. [Google Scholar]
- Novak, P.; Vokralova, J.; Broucek, J. Effects of the stage and number of lactation on milk yield of dairy cows kept in open barn during high temperatures in summer months. Arch. Anim. Breed. 2009, 52, 574–586. [Google Scholar] [CrossRef]
- Neves, M.L.M.W.; Azevedo, M.D.; Guim, A.; Leite, A.M.; Chagas, J.C. Critical levels of the thermal comfort index for Santa Ines sheep under grazing at the agreste region of Pernambuco State. Acta Sci. Anim. Sci. 2009, 31, 169–175. [Google Scholar] [CrossRef]
- Paim, T.P.; Borges, B.O.; Lima, P.M.T. Relation between thermographic temperatures of lambs and thermal comfort indices. Int. J. Appl. Anim. Sci. 2012, 1, 108–115. [Google Scholar]
- Finocchiaro, R.; van Kaam, J.B.; Portolano, B.; Misztal, I. Effect of heat stress on production of Mediterranean dairy sheep. J. Dairy Sci. 2005, 88, 1855–1864. [Google Scholar] [CrossRef]
- Sevi, A.; Annicchiarico, G.; Albenzio, M.; Taibi, L.; Muscio, A.; Dell’Aquila, S. Effects of solar radiation and feeding time on behavior immune response and production of lactating ewes under high ambient temperature. J. Dairy Sci. 2001, 84, 629–640. [Google Scholar] [CrossRef]
- Schaefer, A.L.; Matthews, L.R.; Cook, N.J.; Webster, J.; Scott, S.L. Novel non-invasive measures of animal welfare. In Proceedings of the Animal Welfare and Behaviour, Science Solution, Joint NAWAC/ISAE Conference, Hamilton, New Zeland, 27–28 June 2002. [Google Scholar]
- Sutherland, M.A.; Worth, G.M.; Dowling, S.K.; Lowe, G.L.; Cave, V.M.; Stewart, M. Evaluation of infrared thermography as a non-invasive method of measuring the autonomic nervous response in sheep. PLoS ONE 2020, 15, e0233558. [Google Scholar] [CrossRef]
- Montanholi, Y.R.; Nicholas, E.O.; Kendall, C.S.; Schenkel, F.S.; Mcbride, B.W.; Miller, S.P. Application of infrared thermography as an indicator of heat and methane production and its use in the study of skin temperature in response to physiological events in dairy cattle (Bos taurus). J. Therm. Biol. 2008, 33, 468–475. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Pereira, A.M.F.; Wang, D.; Martínez-Burnes, J.; Ghezzi, M.; Hernández-Avalos, I.; Lendez, P.; Mora-Medina, P.; Casas, A.; Olmos-Hernández, A.; et al. Clinical Applications and Factors Involved in Validating Thermal Windows Used in Infrared Thermography in Cattle and River Buffalo to Assess Health and Productivity. Animals 2021, 11, 2247. [Google Scholar] [CrossRef]
- Verduzco-Mendoza, A.; Bueno-Nava, A.; Wang, D.; Martínez-Burnes, J.; Olmos-Hernández, A.; Casas, A.; Domínguez, A.; Mota-Rojas, D. Experimental Applications and Factors Involved in Validating Thermal Windows Using Infrared Thermography to Assess the Health and Thermostability of Laboratory Animals. Animals 2021, 11, 3448. [Google Scholar] [CrossRef] [PubMed]
- Martello, L.S.; Da Luz, S.S.; Gomes, R.C.; Corte, R.S.R.P.; Leme, P.R. Infrared thermography as a tool to evaluate body surface temperature and its relationship with feed efficiency in Bos indicus cattle in tropical conditions. Int. J. Biometeorol. 2016, 60, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.J.; Kennedy, A.D.; Scott, S.L.; Kyle, B.L.; Shaefer, A.L. Daily variation in the udder surface temperature of dairy cows measured by infrared thermography: Potential for mastitis detection. Can. J. Anim. Sci. 2003, 83, 687–693. [Google Scholar] [CrossRef]
- Starling, J.M.C.; Silva, R.G.D.; Cerón-Muñoz, M.; Barbosa, G.S.S.C.; Costa, M.J.R.P.D. Analysis of some physiological variables for the evaluation of the degree of adaptation in sheep submitted to heat stress. R. Bras. Zootec. 2002, 31, 2070–2077. [Google Scholar] [CrossRef]
- Paim, T.P.; Martins, R.F.S.; Cardoso, C.; Dallago, B.; Louvandini, H.; McManus, C. Thermal comfort index and infrared temperatures for lambs subjected to different environmental conditions. Sci. Agric. 2014, 71, 356–361. [Google Scholar] [CrossRef]
- Salles, M.S.V.; da Silva, S.C.; Salles, F.A.; Roma, L.C.; El Faro, L.; Mac Lean, P.A.B.; de Oliveira, C.E.L.; Martello, L.S. Mapping the body surface temperature of cattle by infrared thermography. J. Therm. Biol. 2016, 62, 63–69. [Google Scholar] [CrossRef]
- Peng, D.; Chen, S.; Li, G.; Chen, J.; Wang, J.; Gu, X. Infrared thermography measured body surface temperature and its relationship with rectal temperature in dairy cows under different temperature-humidity indexes. Int. J. Biometeorol. 2019, 63, 327–336. [Google Scholar] [CrossRef]
- Tadesse, D.; Patra, A.K.; Puchala, R.; Goetsch, A.L. Effects of High Heat Load Conditions on Blood Constituent Concentrations in Dorper, Katahdin, and St. Croix Sheep from Different Regions of the USA. Animals 2022, 12, 2273. [Google Scholar] [CrossRef]
- Sejian, V.; Maurya, V.P.; Kumar, K.; Naqvi, S.M.K. Effect of multiple stresses on growth and adaptive capability of Malpura ewes under semi-arid tropical environment. Trop. Anim. Health Prod. 2012, 45, 107–116. [Google Scholar] [CrossRef]
- Indu, S.; Sejian, V.; Naqvi, S.M.K. Impact of stimulated heat stress on growth, physiological adaptability, blood metabolites and endocrine responses in Malpura ewes under semiarid tropical environment. Anim. Prod. Sci. 2014, 55, 766–776. [Google Scholar] [CrossRef]
- III, J.W.C.; Krausman, P.R.; Rosenstock, S.S.; Turner, J.C. Mechanisms of thermoregulation and water balance in desert ungulates. Wildl. Soc. Bull. 2006, 34, 570–581. [Google Scholar] [CrossRef]
- Indu, S.; Sejian, V.; Kumar, D.; Pareek, A.; Naqvi, S.M.K. Ideal proportion of roughage and concentrate required for Malpura ewes to adapt and reproduce under semi-arid tropical environment. Trop. Anim. Health. Prod. 2015, 47, 1487–1495. [Google Scholar] [CrossRef]
- Mahjoubi, E.; Amanlou, H.; Mirzaei-Alamouti, H.R.; Aghaziarati, N.; Yazdi, M.H.; Noori, G.R.; Yuan, K.; Baumgard, L.H. The effect of cyclical and mild heat stress on productivity and metabolism in Afshari lambs. J. Anim. Sci. 2014, 92, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Baumgard, L.H.; Rhoads, R.P. Effects of heat stress on postabsorptive metabolism and energetics. Annu. Rev. Anim. Biosci. 2013, 1, 311–337. [Google Scholar] [CrossRef] [PubMed]
- Rhoads, M.L.; Rhoads, R.P.; VanBaale, M.J.; Collier, R.J.; Sanders, S.R.; Weber, W.J.; Crooker, B.A.; Baumgard, L.H. Effects of heat stress and plane of nutrition on lactating Holstein cows: I. Production, metabolism, and aspects of circulating somatotropin. J. Dairy Sci. 2009, 92, 1986–1997. [Google Scholar] [CrossRef]
- Baumgard, L.H.; Rhoads, R.P. Ruminant nutrition symposium: Ruminant production and metabolic responses to heat stress. J. Anim. Sci. 2012, 90, 1855–1865. [Google Scholar] [CrossRef]
- Salem, J.A.; Kobeisy, M.A.; Zenhom, M.; Hayder, M. Effect of season and ascorbic acid supplementation on some blood constituents of suckling Chois lambs and its crosses with Ossimi sheep in Upper Egypt. Assiut J. Agric. Sci. 1998, 29, 87–100. [Google Scholar]
- Marai, I.F.M.; Daader, A.H.; Makkawy, M.Y.; Gabr, H.A.; Ibrahim, H. Effect of housing system, season of year and age on some physiological parameters and blood constituents of Ossimi sheep. J. Arid Environ. 1992, 22, 277–285. [Google Scholar] [CrossRef]
- Srikandakumar, A.; Johnson, E.H.; Mahgoub, O. Effect of heat stress on respiratory rate, rectal temperature and blood chemistry in Omani and Australian Merino sheep. Small Rumin. Res. 2003, 49, 193–198. [Google Scholar] [CrossRef]
- Badakhshan, Y.; Mirmahmoudi, R. Blood metabolites of one-humped camel (Camelus dromedarius) versus sheep during summer heat stress. Iran. J. Vet. Med. 2016, 10, 65–71. [Google Scholar] [CrossRef]
- Nazifi, S.; Saeb, M.; Rowghani, E.; Kaveh, K. The influences of thermal stress on serum biochemical parameters of Iranian fat-tailed sheep and their correlation with triiodothyronine (T3), thyroxine (T4) and cortisol concentrations. Comp. Clin. Path. 2003, 12, 135. [Google Scholar] [CrossRef]
- Rathwa, S.D.; Vasava, A.A.; Pathan, M.M.; Madhira, S.P.; Patel, Y.G.; Pande, A.M. Effect of season on physiological, biochemical, hormonal, and oxidative stress parameters of indigenous sheep. Vet. World 2017, 10, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Wojtas, K.; Cwynar, P.; Kolacz, R. Effect of thermal stress on physiological and blood parameters in merino sheep. Bull. Vet. Inst. Pulawy. 2014, 58, 283–288. [Google Scholar] [CrossRef]
- Hrkovic-Porobija, A.; Hodzic, A.; Hadzimusic, N. Functional liver stress in dairy sheep. Indian J. Small Rumin. 2017, 23, 194–197. [Google Scholar] [CrossRef]
- Baumgartner, W.; Parnthaner, A. Influence of age, season and pregnancy upon blood parameters in Austrian Karakul sheep. Small Rumin. Res. 1994, 11, 147–151. [Google Scholar] [CrossRef]
- Marai, I.F.M.; Habeeb, A.A.M.; Daader, A.H.; Yousef, H.M. Effects of Egyptian subtropical conditions and the heat stress alleviation techniques of water spray and diaphoretics on the growth and physiological functions of Friesian calves. J. Arid. Environ. 1995, 30, 219–225. [Google Scholar] [CrossRef]
- Habeeb, A.A.; El-Masry, K.A.; Aboul-Naga, A.I.; Kamal, T.H. The effect of hot summer climate and level of milk yield on blood biochemistry and circulating thyroid and progesterone hormones in Friesian cows. Arab. J. Nucl. Sci. Appl. 1996, 29, 161–173. [Google Scholar]
- Macías-Cruz, U.; Correa-Calderón, A.; Mellado, M.; Meza-Herrera, C.A.; Aréchiga, C.F.; Avendaño-Reyes, L. Thermoregulatory response to outdoor heat stress of hair sheep females at different physiological state. Int. J. Biometeorol. 2018, 62, 2151–2160. [Google Scholar] [CrossRef]
- Karthik, D.; Suresh, J.; Sharma, G.; Ramana, J.; Gangaraju, G.; Reddy, P.P.R.; Reddy, Y.P.K.; Yasaswini, D.; Adegbeye, M. Adaptive profiles of Nellore sheep with reference to farming system and season: Physiological, hemato-biochemical, hormonal, oxidative-enzymatic and reproductive standpoint. Heliyon 2021, 7, e07117. [Google Scholar] [CrossRef]
- Stewart, M.; Webster, J.R.; Verkerk, G.A.; Schaefer, A.L.; Colyn, J.J.; Stafford, K.J. Non-invasive measurement of stress in dairy cows using infrared thermography. Physiol. Behav. 2007, 92, 520–525. [Google Scholar] [CrossRef]
- Tan, J.H.; Ng, E.Y.K.; Acharya, U.R.; Chee, C. Infrared thermography on ocular surface temperature: A review. Infrared Phys. Technol. 2009, 52, 97–108. [Google Scholar] [CrossRef]
- Meyer, C.W.; Ootsuka, Y.; Romanovsky, A.A. Body Temperature Measurements for Metabolic Phenotyping in Mice. Front. Physiol. 2017, 8, 520. [Google Scholar] [CrossRef]
- Joy, A.; Taheri, S.; Dunshea, F.R.; Leury, B.J.; DiGiacomo, K.; Osei-Amponsah, R.; Brodie, G.; Chauhan, S.S. Non-invasive measure of heat stress in sheep using machine learning techniques and infrared thermography. Small Rumin. Res. 2022, 207, 106592. [Google Scholar] [CrossRef]







| Temperature | Period 1 | Period 2 | Period 3 | Period | Housing | Period × Housing | |||
|---|---|---|---|---|---|---|---|---|---|
| Ext | Int | Ext | Int | Ext | Int | ||||
| Rectal temp. (RT) | 39.1 ± 0.35 A | 39.7 ± 0.35 B | 39.6 ± 0.21 B | 39.5 ± 0.21 B | 39.2 ± 0.26 A | 39.5 ± 0.23 B | 0.017 | 0.000 | 0.000 |
| Nose temp. (NT) | 31.3 ± 3.32 A | 35.3 ± 2.5 B | 36.5 ± 1.71 C | 34.8 ± 2.46 B | 31.6 ± 2.42 A | 33.1 ± 2.6 B | 0.000 | 0.017 | 0.000 |
| Eye temp. (ET) | 36.8 ± 1.3 A | 39.3 ± 0.91 B | 39.8 ± 1 B | 38.1 ± 0.71 C | 37.8 ± 1.19 C | 38.7 ± 1.18 B | 0.002 | 0.014 | 0.000 |
| Leg temp. (LT) | 18.3 ± 3.26 A | 24.7 ± 2.61 B | 34.2 ± 2.33 C | 32.7 ± 2 D | 28.1 ± 2.44 E | 30.6 ± 2.26 F | 0.000 | 0.000 | 0.000 |
| Abdomen temp. (AT) | 16.01 ± 3 A | 23.4 ± 1.84 B | 36.4 ± 2.21 C | 32.7 ± 1.09 D | 26.9 ± 3 E | 30.1 ± 2.19 F | 0.000 | 0.000 | 0.000 |
| Temperature | Period 1 | Period 2 | Period 3 | Period | Breeding | Period × Breeding | |||
|---|---|---|---|---|---|---|---|---|---|
| Ext | Int | Ext | Int | Ext | Int | ||||
| CORT * (nmol/L) | 77.9 ± 25.1 A | 48.14 ± 17 B | 117.5 ± 14 C | 97.6 ± 11.8 D | 95.8 ± 22.1 D | 77.7 ± 11.2 A | 0.000 | 0.000 | 0.352 |
| T3 (nmol/L) | 1.43 ± 0.32 A | 1.53 ± 0.3 A | 0.51 ± 0.1 B | 0.62 ± 0.1 C | 0.76 ± 0.2 C | 0.89 ± 0.2 D | 0.000 | 0.012 | 0.957 |
| T4 (nmol/L) | 41 ± 4.13 A | 44.27 ± 5.5 A | 18.47 ± 1.9 B | 22.13 ± 2.7 C | 27.38 ± 2.8 D | 29.47 ± 3.7 D | 0.000 | 0.000 | 0.660 |
| INS (mU/L) | 237.9 ± 33 A | 231.1 ± 28 A | 299.2 ± 40 B | 265.4 ± 33 C | 279.6 ± 39 C | 272.9 ± 33 C | 0.000 | 0.027 | 0.194 |
| NEFA (mmol/L) | 0.43 ± 0.1 A | 0.43 ± 0.06 A | 0.3 ± 0.07 B | 0.32 ± 0.1 B | 0.37 ± 0.05 C | 0.41 ± 0.07 D | 0.000 | 0.217 | 0.509 |
| BHB (mmol/L) | 0.58 ± 0.1 A | 0.65 ± 0.1 A | 0.32 ± 0.13 B | 0.33 ± 0.1 C | 0.36 ± 0.12 D | 0.34 ± 0.09 E | 0.000 | 0.295 | 0.245 |
| GLU (mmol/L) | 3.12 ± 0.3 A | 3.5 ± 0.43 B | 2.37 ± 0.18 C | 2.69 ± 0.37 D | 2.73 ± 0.4 D | 2.87 ± 0.4 D | 0.000 | 0.000 | 0.366 |
| Ca (mmol/L) | 2.95 ± 0.12 A | 2.68 ± 0.37 B | 2.66 ± 0.14 B | 2.47 ± 0.31 C | 2.66 ± 0.26 B | 2.61 ± 0.3 B | 0.001 | 0.002 | 0.250 |
| P (mmol/L) | 2.08 ± 0.24 A | 2.04 ± 0.26 A | 1.86 ± 0.24 B | 1.9 ± 0.2 B | 1.95 ± 0.22 B | 1.89 ± 0.24 B | 0.004 | 0.665 | 0.596 |
| Mg (mmol/L) | 1.29 ± 0.11 A | 1.26 ± 0.1 A | 1.22 ± 0.07 B | 1.2 ± 0.12 B | 1.25 ± 0.11 B | 1.17 ± 0.1 C | 0.017 | 0.040 | 0.375 |
| TPROT (g/L) | 64.77 ± 2.6 A | 65.4 ± 2.7 A | 72.95 ± 3.4 B | 73.01 ± 2.6 B | 70.17 ± 2.7 C | 70.22 ± 3 C | 0.000 | 0.665 | 0.895 |
| ALB (g/L) | 33.34 ± 1.6 A | 32.12 ± 1.8 A | 40.01 ± 1.8 B | 38.08 ± 1.9 C | 37.37 ± 1.7 D | 36.12 ± 1.3 E | 0.000 | 0.040 | 0.894 |
| GLB (g/L) | 31.44 ± 3.1 A | 33.28 ± 2.1 A | 32.94 ± 4 A | 34.93 ± 3.9 B | 32.8 ± 2.9 A | 34.1 ± 3.5 B | 0.358 | 0.064 | 0.948 |
| Urea (mmol/L) | 4.58 ± 0.4 A | 4.11 ± 0.67 A | 5.41 ± 0.53 B | 5.15 ± 0.7 C | 4.87 ± 0.6 A | 4.21 ± 0.6 D | 0.000 | 0.000 | 0.410 |
| TGC (mmol/L) | 0.11 ± 0.03A | 0.16 ± 0.05 B | 0.09 ± 0.03 C | 0.13 ± 0.04 D | 0.1 ± 0.03 A | 0.14 ± 0.05 D | 0.111 | 0.000 | 0.895 |
| CHOL (mmol/L) | 1.30 ± 0.2 A | 1.65 ± 0.4 B | 0.97 ± 0.2 C | 1.3 ± 0.31 A | 1.21 ± 0.21 A | 1.52 ± 0.4 B | 0.000 | 0.000 | 0.968 |
| TBIL (µmol/L) | 3.15 ± 0.8 A | 3.48 ± 0.6 A | 4.1 ± 0.83 B | 4.24 ± 0.75 B | 3.31 ± 0.81 A | 3.35 ± 0.54 A | 0.000 | 0.259 | 0.719 |
| DBIL (µmol/L) | 1.24 ± 0.44 A | 1.43 ± 0.36 A | 1.69 ± 0.6 B | 1.73 ± 0.44 B | 1.36 ± 0.51 C | 1.43 ± 0.4 A | 0.003 | 0.288 | 0.793 |
| IBIL (µmol/L) | 1.91 ± 0.65 A | 2.05 ± 0.5 A | 2.4 ± 0.45 B | 2.51 ± 0.8 B | 1.95 ± 0.63 A | 1.92 ± 0.36 A | 0.001 | 0.553 | 0.814 |
| CR (U/L) | 88 ± 10.4 A | 94.4 ± 6.66 A | 110 ± 13 B | 106.6 ± 7.6 B | 106.3 ± 9.1 C | 97.6 ± 10.1 B | 0.000 | 0.332 | 0.008 |
| AST (U/L) | 50.42 ± 9.7 A | 53.7 ± 12.8 A | 61 ± 10.2 B | 63.3 ± 16.5 B | 58.12 ± 11 C | 58 ± 14.2 C | 0.006 | 0.474 | 0.857 |
| ALT (U/L) | 34.51 ± 2.3 A | 35.1 ± 2.07 A | 42.65 ± 3.1 B | 40.6 ± 2.7 B | 38.56 ± 2.5 C | 39.05 ± 2.4 C | 0.000 | 0.508 | 0.061 |
| GGT (U/L) | 53.9 ± 13.3 A | 57.23 ± 16 A | 66 ± 18.6 B | 64.9 ± 16.2 B | 62.41 ± 17 B | 63.31 ± 16 B | 0.042 | 0.748 | 0.856 |
| LDH (U/L) | 129 ± 10.6 A | 126.3 ± 17 A | 160.5 ± 14 B | 148.8 ± 18 C | 139.1 ± 16 C | 144.1 ± 16 C | 0.000 | 0.276 | 0.092 |
| ALP (U/L) | 118 ± 23.9 A | 121.4 ± 14 A | 144.8 ± 28 B | 141.9 ± 19 B | 131.4 ± 24A | 135.7 ± 20 B | 0.000 | 0.727 | 0.771 |
| CK (U/L) | 165.4 ± 51 A | 149.9 ± 11 A | 198.4 ± 20 B | 193.3 ± 37 B | 167.7 ± 14 C | 163.2 ± 9.3 C | 0.000 | 0.143 | 0.676 |
| HOMA | 4.77 ± 0.85 A | 5.2 ± 1.1 A | 4.55 ± 0.75 A | 4.62 ± 1.07 A | 4.87 ± 0.9 A | 5.02 ± 1.02 A | 0.162 | 0.260 | 0.723 |
| QUICKI | 0.31 ± 0.01 A | 0.3 ± 0.01 A | 0.31 ± 0.01 A | 0.31 ± 0.01 A | 0.3 ± 0.01 A | 0.3 ± 0.01 A | 0.130 | 0.373 | 0.694 |
| RQUICKI | 0.34 ± 0.01 A | 0.34 ± 0.01 A | 0.37 ± 0.02 B | 0.37 ± 0.03 B | 0.35 ± 0.01 C | 0.34 ± 0.01 A | 0.000 | 0.246 | 0.906 |
| RQUICKIBHB | 0.38 ± 0.01 A | 0.36 ± 0.02 B | 0.46 ± 0.05 C | 0.45 ± 0.05 C | 0.42 ± 0.03 D | 0.41 ± 0.03 E | 0.000 | 0.158 | 0.937 |
| Rectal Temperature (RT) | Nose Temperature (NT) | Eye Temperature (ET) | Leg Temperature (LT) | Abdomen Temperature (AT) | |
|---|---|---|---|---|---|
| COR 1 | −0.109 | −0.005 | 0.040 | 0.448 ** | 0.474 ** |
| T3 | −0.082 | −0.196 | −0.247 * | −0.698 ** | −0.723 ** |
| T4 | −0.091 | −0.229 * | −0.224 * | −0.760 ** | −0.776 ** |
| INS | 0.096 | 0.153 | 0.099 | 0.445 ** | 0.427 ** |
| NEFA | −0.104 | −0.143 | −0.226 * | −0.451 ** | −0.468 ** |
| BHB | −0.046 | −0.055 | −0.107 | −0.627 ** | −0.632 ** |
| GLU | −0.026 | −0.208 * | −0.259 ** | −0.516 ** | −0.547 ** |
| Ca | −0.260 ** | −0.195 | −0.254 * | −0.447 ** | −0.416 ** |
| P | −0.028 | −0.105 | −0.156 | −0.269 ** | −0.216 * |
| Mg | −0.051 | −0.022 | −0.072 | −0.315 ** | −0.259 ** |
| TPROT | 0.005 | 0.152 | 0.123 | 0.651 ** | 0.656 ** |
| ALB | 0.037 | 0.153 | 0.172 | 0.449 ** | 0.492 ** |
| GLB | −0.032 | −0.003 | −0.049 | 0.185 | 0.149 |
| Urea | −0.051 | 0.055 | 0.033 | 0.319 ** | 0.321 ** |
| TGC | 0.225* | 0.125 | 0.080 | −0.058 | −0.024 |
| CHOL | 0.105 | 0.010 | −0.062 | −0.233 * | −0.225 * |
| TBIL | 0.074 | 0.331 ** | 0.140 | 0.321 ** | 0.372 ** |
| DBIL | 0.183 | 0.328 ** | 0.092 | 0.268 ** | 0.325 ** |
| IBIL | −0.045 | 0.183 | 0.114 | 0.218 * | 0.242 * |
| CR | 0.196 | 0.317 ** | 0.183 | 0.550 ** | 0.572 ** |
| AST | 0.036 | 0.149 | 0.178 | 0.232 * | 0.324 ** |
| ALT | 0.111 | 0.355 ** | 0.269 ** | 0.666 ** | 0.693 ** |
| GGT | 0.103 | 0.090 | 0.145 | 0.283 ** | 0.292 ** |
| LDH | 0.101 | 0.214 * | 0.212 * | 0.471 ** | 0.554 ** |
| ALP | −0.041 | 0.044 | 0.207 * | 0.366 ** | 0.350 ** |
| CK | 0.126 | 0.305 ** | 0.177 | 0.290 ** | 0.384 ** |
| HOMA | 0.048 | −0.072 | −0.170 | −0.089 | −0.134 |
| QUICKI | −0.029 | 0.089 | 0.160 | 0.105 | 0.139 |
| RQUICKI | 0.078 | 0.183 | 0.270 ** | 0.386 ** | 0.403 ** |
| RQUICKIBHB | 0.126 | 0.183 | 0.277 ** | 0.625 ** | 0.639 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Čukić, A.; Rakonjac, S.; Djoković, R.; Cincović, M.; Bogosavljević-Bošković, S.; Petrović, M.; Savić, Ž.; Andjušić, L.; Andjelić, B. Influence of Heat Stress on Body Temperatures Measured by Infrared Thermography, Blood Metabolic Parameters and Its Correlation in Sheep. Metabolites 2023, 13, 957. https://doi.org/10.3390/metabo13080957
Čukić A, Rakonjac S, Djoković R, Cincović M, Bogosavljević-Bošković S, Petrović M, Savić Ž, Andjušić L, Andjelić B. Influence of Heat Stress on Body Temperatures Measured by Infrared Thermography, Blood Metabolic Parameters and Its Correlation in Sheep. Metabolites. 2023; 13(8):957. https://doi.org/10.3390/metabo13080957
Chicago/Turabian StyleČukić, Aleksandar, Simeon Rakonjac, Radojica Djoković, Marko Cincović, Snežana Bogosavljević-Bošković, Milun Petrović, Željko Savić, Ljiljana Andjušić, and Biljana Andjelić. 2023. "Influence of Heat Stress on Body Temperatures Measured by Infrared Thermography, Blood Metabolic Parameters and Its Correlation in Sheep" Metabolites 13, no. 8: 957. https://doi.org/10.3390/metabo13080957
APA StyleČukić, A., Rakonjac, S., Djoković, R., Cincović, M., Bogosavljević-Bošković, S., Petrović, M., Savić, Ž., Andjušić, L., & Andjelić, B. (2023). Influence of Heat Stress on Body Temperatures Measured by Infrared Thermography, Blood Metabolic Parameters and Its Correlation in Sheep. Metabolites, 13(8), 957. https://doi.org/10.3390/metabo13080957