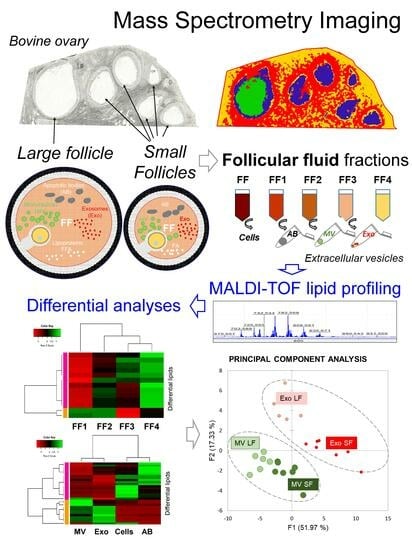
Extracellular Vesicles Contribute to the Difference in Lipid Composition between Ovarian Follicles of Different Size Revealed by Mass Spectrometry Imaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Chemicals
2.3. Biological Materials
2.4. Mass Spectrometry Imaging (MSI) by MALDI-TOF
2.5. Transmission Electron Microscopy (TEM) Analyses of Follicular Fluid Extracellular Vesicles
2.6. Lipid Profiling of Follicular Fluid Fractions by MALDI-TOF MS
2.7. Lipid Annotation
2.8. Pathway Analysis
3. Results
3.1. Mass Spectrometry Imaging (MSI) on Ovarian Sections Confirmed the Differences in Follicular Fluid Lipid Abundances between the Follicles in the Same Ovary
3.2. Analysis of Lipids in Depleted Follicular Fluid Fractions
3.3. Analysis of Lipids in Cellular and Vesicle Fractions of Follicular Fluid
3.4. Analysis of Lipids in Follicular Fluid Extracellular Vesicles
3.5. Comparative Analysis of Lipids between MVs and Exo
3.6. Comparative Analysis of ffEV Lipid Abundance between the Large and Small Follicles
3.7. Comparative Analysis and Annotation of Lipids in MVs and Exo from the Large and Small Follicles
4. Discussion
4.1. Mass Spectrometry Imaging of Bovine Ovary Revealed the Differences of Lipid Composition between the Large and Small Follicles
4.2. Lipid Distribution in Different Fractions of Follicular Fluid
4.3. Lipids in MVs and Exo-Enriched Fractions Differed between the Large and Small Follicles
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dalbies-Tran, R.; Cadoret, V.; Desmarchais, A.; Elis, S.; Maillard, V.; Monget, P.; Monniaux, D.; Reynaud, K.; Saint-Dizier, M.; Uzbekova, S. A comparative analysis of oocyte development in mammals. Cells 2020, 9, 1002. [Google Scholar] [CrossRef] [PubMed]
- Dunning, K.R.; Russell, D.L.; Robker, R.L. Lipids and oocyte developmental competence: The role of fatty acids and b-oxidation. Reproduction 2014, 148, R15–R27. [Google Scholar] [CrossRef] [PubMed]
- Collado-Fernandez, E.; Picton, H.M.; Dumollard, R. Metabolism throughout follicle and oocyte development in mammals. Int. J. Dev. Biol. 2012, 56, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Yanez-Mo, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borras, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed]
- Saeed-Zidane, M.; Linden, L.; Salilew-Wondim, D.; Held, E.; Neuhoff, C.; Tholen, E.; Hoelker, M.; Schellander, K.; Tesfaye, D. Cellular and exosome mediated molecular defense mechanism in bovine granulosa cells exposed to oxidative stress. PLoS ONE 2017, 12, e0187569. [Google Scholar] [CrossRef]
- Tesfaye, D.; Hailay, T.; Salilew-Wondim, D.; Hoelker, M.; Bitseha, S.; Gebremedhn, S. Extracellular vesicle mediated molecular signaling in ovarian follicle: Implication for oocyte developmental competence. Theriogenology 2020, 150, 70–74. [Google Scholar] [CrossRef]
- Skotland, T.; Sagini, K.; Sandvig, K.; Llorente, A. An emerging focus on lipids in extracellular vesicles. Adv. Drug Deliv. Rev. 2020, 159, 308–321. [Google Scholar] [CrossRef]
- Lonergan, P.; Monaghan, P.; Rizos, D.; Boland, M.P.; Gordon, I. Effect of follicle size on bovine oocyte quality and developmental competence following maturation, fertilization, and culture in vitro. Mol. Reprod. Dev. 1994, 37, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Blondin, P.; Sirard, M.A. Oocyte and follicular morphology as determining characteristics for developmental competence in bovine oocytes. Mol. Reprod. Dev. 1995, 41, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Monniaux, D.; Cadoret, V.; Clément, F.; Dalbies Tran, R.; Elis, S.; Fabre, S.; Maillard, V.; Monget, P.; Uzbekova, S. Folliculogenesis. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–22. ISBN 978-0-12-801238-3. [Google Scholar]
- Brantmeier, S.A.; Grummer, R.R.; Ax, R.L. Concentrations of high density lipoproteins vary among follicular sizes in the bovine. J. Dairy Sci. 1987, 70, 2145–2149. [Google Scholar] [CrossRef]
- Leroy, J.L.; Vanholder, T.; Delanghe, J.R.; Opsomer, G.; Van Soom, A.; Bols, P.E.; de Kruif, A. Metabolite and ionic composition of follicular fluid from different-sized follicles and their relationship to serum concentrations in dairy cows. Anim. Reprod. Sci. 2004, 80, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Renaville, B.; Bacciu, N.; Comin, A.; Motta, M.; Poli, I.; Vanini, G.; Prandi, A. Plasma and follicular fluid fatty acid profiles in dairy cows. Reprod. Domest. Anim. 2010, 45, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Bertevello, P.S.; Teixeira-Gomes, A.P.; Labas, V.; Cordeiro, L.; Blache, M.C.; Papillier, P.; Singina, G.; Uzbekov, R.; Maillard, V.; Uzbekova, S. Maldi-tof mass spectrometry revealed significant lipid variations in follicular fluid and somatic follicular cells but not in enclosed oocytes between the large dominant and small subordinate follicles in bovine ovary. Int. J. Mol. Sci. 2020, 21, 6661. [Google Scholar] [CrossRef] [PubMed]
- Navakanitworakul, R.; Hung, W.T.; Gunewardena, S.; Davis, J.S.; Chotigeat, W.; Christenson, L.K. Characterization and small rna content of extracellular vesicles in follicular fluid of developing bovine antral follicles. Sci. Rep. 2016, 6, 25486. [Google Scholar] [CrossRef]
- Bertevello, P.S.; Teixeira-Gomes, A.P.; Seyer, A.; Vitorino Carvalho, A.; Labas, V.; Blache, M.C.; Banliat, C.; Cordeiro, L.A.V.; Duranthon, V.; Papillier, P.; et al. Lipid identification and transcriptional analysis of controlling enzymes in bovine ovarian follicle. Int J Mol Sci 2018, 19, 3261. [Google Scholar] [CrossRef]
- Baker, T.C.; Han, J.; Borchers, C.H. Recent advancements in matrix-assisted laser desorption/ionization mass spectrometry imaging. Curr. Opin. Biotechnol. 2017, 43, 62–69. [Google Scholar] [CrossRef]
- Perry, W.J.; Patterson, N.H.; Prentice, B.M.; Neumann, E.K.; Caprioli, R.M.; Spraggins, J.M. Uncovering matrix effects on lipid analyses in maldi imaging mass spectrometry experiments. J. Mass Spectrom. 2020, 55, e4491. [Google Scholar] [CrossRef]
- Campbell, D.I.; Ferreira, C.R.; Eberlin, L.S.; Cooks, R.G. Improved spatial resolution in the imaging of biological tissue using desorption electrospray ionization. Anal. Bioanal. Chem. 2012, 404, 389–398. [Google Scholar] [CrossRef]
- Uzbekova, S.; Elis, S.; Teixeira-Gomes, A.P.; Desmarchais, A.; Maillard, V.; Labas, V. Maldi mass spectrometry imaging of lipids and gene expression reveals differences in fatty acid metabolism between follicular compartments in porcine ovaries. Biology 2015, 4, 216–236. [Google Scholar] [CrossRef]
- Cordeiro, F.B.; Jarmusch, A.K.; Leon, M.; Ferreira, C.R.; Pirro, V.; Eberlin, L.S.; Hallett, J.; Miglino, M.A.; Cooks, R.G. Mammalian ovarian lipid distributions by desorption electrospray ionization-mass spectrometry (desi-ms) imaging. Anal. Bioanal. Chem. 2020, 412, 1251–1262. [Google Scholar] [CrossRef]
- Leroy, J.L.; Vanholder, T.; Mateusen, B.; Christophe, A.; Opsomer, G.; de Kruif, A.; Genicot, G.; Van Soom, A. Non-esterified fatty acids in follicular fluid of dairy cows and their effect on developmental capacity of bovine oocytes in vitro. Reproduction 2005, 130, 485–495. [Google Scholar] [CrossRef] [PubMed]
- McNatty, K.P.; Heath, D.A.; Henderson, K.M.; Lun, S.; Hurst, P.R.; Ellis, L.M.; Montgomery, G.W.; Morrison, L.; Thurley, D.C. Some aspects of thecal and granulosa cell function during follicular development in the bovine ovary. J. Reprod. Fertil. 1984, 72, 39–53. [Google Scholar] [CrossRef]
- Monniaux, D.; Huet, C.; Besnard, N.; Clement, F.; Bosc, M.; Pisselet, C.; Monget, P.; Mariana, J.C. Follicular growth and ovarian dynamics in mammals. J. Reprod. Fertil. Suppl. 1997, 51, 3–23. [Google Scholar]
- Argov, N.; Sklan, D. Expression of mrna of lipoprotein receptor related protein 8, low density lipoprotein receptor, and very low density lipoprotein receptor in bovine ovarian cells during follicular development and corpus luteum formation and regression. Mol. Reprod. Dev. 2004, 68, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Uzbekova, S.; Bertevello, P.S.; Dalbies-Tran, R.; Elis, S.; Labas, V.; Monget, P.; Teixeira-Gomes, A.-P. Metabolic exchanges between the oocyte and its environment: Focus on lipids. Reprod. Fertil. Dev. 2022, 34, 1–26. [Google Scholar] [CrossRef]
- Guo, X.; Wang, X.; Di, R.; Liu, Q.; Hu, W.; He, X.; Yu, J.; Zhang, X.; Zhang, J.; Broniowska, K.; et al. Metabolic effects of fecb gene on follicular fluid and ovarian vein serum in sheep (ovis aries). Int. J. Mol. Sci. 2018, 19, 539. [Google Scholar] [CrossRef]
- Conti, M.; Hsieh, M.; Zamah, A.M.; Oh, J.S. Novel signaling mechanisms in the ovary during oocyte maturation and ovulation. Mol. Cell. Endocrinol. 2012, 356, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Vigneron, C.; Perreau, C.; Dupont, J.; Uzbekova, S.; Prigent, C.; Mermillod, P. Several signaling pathways are involved in the control of cattle oocyte maturation. Mol. Reprod. Dev. 2004, 69, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Schiller, J.; Süß, R.; Arnhold, J.; Fuchs, B.; Leßig, J.; Müller, M.; Petković, M.; Spalteholz, H.; Zschörnig, O.; Arnold, K. Matrix-assisted laser desorption and ionization time-of-flight (maldi-tof) mass spectrometry in lipid and phospholipid research. Prog. Lipid Res. 2004, 43, 449–488. [Google Scholar] [CrossRef]
- Fuchs, B.; Schiller, J. Maldi-tof ms analysis of lipids from cells, tissues and body fluids. Lipids Health Dis. 2008, 49, 541–565. [Google Scholar]
- Lagarrigue, M.; Lavigne, R.; Chaurand, P.; Com, E.; Pineau, C.; Guével, B. Matrix-assisted laser desorption/ionization imaging mass spectrometry: A promising technique for reproductive research1. Biol. Reprod. 2011, 86, 74. [Google Scholar] [CrossRef]
- Soler, L.; Uzbekova, S.; Blesbois, E.; Druart, X.; Labas, V. Intact cell maldi-tof mass spectrometry, a promising proteomic profiling method in farm animal clinical and reproduction research. Theriogenology 2020, 150, 113–121. [Google Scholar] [CrossRef]
- Thompson, J.E. Matrix-assisted laser desorption ionization-time-of-flight mass spectrometry in veterinary medicine: Recent advances (2019–present). Vet. World 2022, 15, 2623–2657. [Google Scholar] [CrossRef]
- Ferreira, C.R.; Saraiva, S.A.; Catharino, R.R.; Garcia, J.S.; Gozzo, F.C.; Sanvido, G.B.; Santos, L.F.A.; Lo Turco, E.G.; Pontes, J.H.F.; Basso, A.C.; et al. Single embryo and oocyte lipid fingerprinting by mass spectrometry. J. Lipid Res. 2010, 51, 1218–1227. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.H.; Fontes, P.K.; Franchi, F.F.; Nogueira, M.F.; Belaz, K.R.; Tata, A.; Eberlin, M.N.; Sudano, M.J.; Barros, C.M.; Castilho, A.C. Lipid profiles of follicular fluid from cows submitted to ovarian superstimulation. Theriogenology 2017, 94, 64–70. [Google Scholar] [CrossRef]
- Cordeiro, F.B.; Montani, D.A.; Pilau, E.J.; Gozzo, F.C.; Fraietta, R.; Turco, E.G.L. Ovarian environment aging: Follicular fluid lipidomic and related metabolic pathways. J. Assist. Reprod. Genet. 2018, 35, 1385–1393. [Google Scholar] [CrossRef] [PubMed]
- Vireque, A.A.; Tata, A.; Roberta, K.; Belaz, A.; Gabriel, J.; Grázia, V.; Santos, F.N.; Arnold, D.R.; Basso, A.C.; Eberlin, M.N.; et al. Maldi mass spectrometry reveals that cumulus cells modulate the lipid profile of in vitro- matured bovine oocytes. Syst. Biol. Reprod. Med. 2017, 63, 86–99. [Google Scholar] [CrossRef]
- Gonçalves, R.F.; Ferreira, M.S.; Oliveira, D.N.d.; Canevarolo, R.; Achilles, M.A.; D’Ercole, D.L.; Bols, P.E.; Visintin, J.A.; Killian, G.J.; Catharino, R.R. Analysis and characterisation of bovine oocyte and embryo biomarkers by matrix-assisted desorption ionisation mass spectrometry imaging. Reprod. Fertil. Dev. 2014, 28, 293. [Google Scholar] [CrossRef] [PubMed]
- Freret, S.; Oseikria, M.; Le Bourhis, D.; Desmarchais, A.; Briant, E.; Desnoes, O.; Dupont, M.; Le Berre, L.; Ghazouani, O.; Bertevello, P.S.; et al. Effects of a n-3 pufa enriched diet on embryo production in dairy cows. Reproduction 2019, 58(1), 71–83. [Google Scholar] [CrossRef]
- Elis, S.; Oseikria, M.; Vitorino Carvalho, A.; Bertevello, P.S.; Corbin, E.; Teixeira-Gomes, A.P.; Lecardonnel, J.; Archilla, C.; Duranthon, V.; Labas, V.; et al. Docosahexaenoic acid mechanisms of action on the bovine oocyte-cumulus complex. J. Ovarian Res. 2017, 10, 74. [Google Scholar] [CrossRef] [PubMed]
- Pfrieger, F.W.; Vitale, N. Cholesterol and the journey of extracellular vesicles. J. Lipid Res. 2018, 59, 2255–2261. [Google Scholar] [CrossRef] [PubMed]
- Elsherbini, A.; Bieberich, E. Chapter five—Ceramide and exosomes: A novel target in cancer biology and therapy. In Advances in Cancer Research; Chalfant, C.E., Fisher, P.B., Eds.; Academic Press: Cambridge, MA, USA, 2018; Volume 140, pp. 121–154. [Google Scholar]
- Jaspard, B.; Barbaras, R.; Manent, J.; Parinaud, J.; Chap, H.; Perret, B. Biochemical characterization of pre-β 1 high-density lipoprotein from human ovarian follicular fluid: Evidence for the presence of a lipid core. Biochemistry 1996, 35, 1352–1357. [Google Scholar] [CrossRef] [PubMed]
- Arias, A.; Quiroz, A.; Santander, N.; Morselli, E.; Busso, D. Implications of high-density cholesterol metabolism for oocyte biology and female fertility. Front. Cell Dev. Biol. 2022, 10, 941539. [Google Scholar] [CrossRef] [PubMed]
- Volpe, A.; Coukos, G.; Uccelli, E.; Droghini, F.; Adamo, R.; Artini, P.G. Follicular fluid lipoproteins in preovulatory period and their relationship with follicular maturation and progesterone production by human granulosa-luteal cells in vivo and in vitro. J. Endocrinol. Investig. 1991, 14, 737–742. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, Y.; Yang, Z.; Xie, Y.; Mo, Z. The effects of cholesterol metabolism on follicular development and ovarian function. Curr. Mol. Med. 2019, 19, 719–730. [Google Scholar] [CrossRef] [PubMed]
- da Silveira, J.C.; Andrade, G.M.; Simas, R.C.; Martins-Junior, H.A.; Eberlin, M.N.; Smith, L.C.; Perecin, F.; Meirelles, F.V. Lipid profile of extracellular vesicles and their relationship with bovine oocyte developmental competence: New players in intra follicular cell communication. Theriogenology 2021, 174, 1–8. [Google Scholar] [CrossRef]
- Haraszti, R.A.; Didiot, M.C.; Sapp, E.; Leszyk, J.; Shaffer, S.A.; Rockwell, H.E.; Gao, F.; Narain, N.R.; DiFiglia, M.; Kiebish, M.A.; et al. High-resolution proteomic and lipidomic analysis of exosomes and microvesicles from different cell sources. J. Extracell. Vesicles 2016, 5, 32570. [Google Scholar] [CrossRef]
- Paolino, G.; Buratta, S.; Mercuri, S.R.; Pellegrino, R.M.; Urbanelli, L.; Emiliani, C.; Bertuccini, L.; Iosi, F.; Huber, V.; Brianti, P.; et al. Lipidic profile changes in exosomes and microvesicles derived from plasma of monoclonal antibody-treated psoriatic patients. Front. Cell Dev. Biol. 2022, 10, 923769. [Google Scholar] [CrossRef]
- Record, M.; Silvente-Poirot, S.; Poirot, M.; Wakelam, M.J.O. Extracellular vesicles: Lipids as key components of their biogenesis and functions. J. Lipid Res. 2018, 59, 1316–1324. [Google Scholar] [CrossRef]
- Skotland, T.; Sandvig, K.; Llorente, A. Lipids in exosomes: Current knowledge and the way forward. Prog. Lipid Res. 2017, 66, 30–41. [Google Scholar] [CrossRef]
- Lee, S.S.; Won, J.H.; Lim, G.J.; Han, J.; Lee, J.Y.; Cho, K.O.; Bae, Y.K. A novel population of extracellular vesicles smaller than exosomes promotes cell proliferation. Cell Commun. Signal. CCS 2019, 17, 95. [Google Scholar] [CrossRef] [PubMed]
- Yuana, Y.; Levels, J.; Grootemaat, A.; Sturk, A.; Nieuwland, R. Co-isolation of extracellular vesicles and high-density lipoproteins using density gradient ultracentrifugation. J. Extracell. Vesicles 2014, 3, 23262. [Google Scholar] [CrossRef]
- Sun, Y.; Saito, K.; Saito, Y. Lipid profile characterization and lipoprotein comparison of extracellular vesicles from human plasma and serum. Metabolites 2019, 9, 259. [Google Scholar] [CrossRef]
- Law, S.H.; Chan, M.L.; Marathe, G.K.; Parveen, F.; Chen, C.H.; Ke, L.Y. An updated review of lysophosphatidylcholine metabolism in human diseases. Int. J. Mol. Sci. 2019, 20, 1149. [Google Scholar] [CrossRef]
- Drzazga, A.; Sowinska, A.; Koziolkiewicz, M. Lysophosphatidylcholine and lysophosphatidylinosiol--novel promissing signaling molecules and their possible therapeutic activity. Acta Pol. Pharm. 2014, 71, 887–899. [Google Scholar] [PubMed]
- Dupont, J.; Reverchon, M.; Cloix, L.; Froment, P.; Rame, C. Involvement of adipokines, ampk, pi3k and the ppar signaling pathways in ovarian follicle development and cancer. Int. J. Dev. Biol. 2012, 56, 959–967. [Google Scholar] [CrossRef]
- Moallem, U. Invited review: Roles of dietary n-3 fatty acids in performance, milk fat composition, and reproductive and immune systems in dairy cattle. J. Dairy Sci. 2018, 101, 8641–8661. [Google Scholar] [CrossRef]
- Elis, S.; Freret, S.; Desmarchais, A.; Maillard, V.; Cognie, J.; Briant, E.; Touze, J.L.; Dupont, M.; Faverdin, P.; Chajes, V.; et al. Effect of a long chain n-3 pufa-enriched diet on production and reproduction variables in holstein dairy cows. Anim. Reprod. Sci. 2016, 164, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.; Kwong, W.Y.; Li, D.; Salter, A.M.; Lea, R.G.; Sinclair, K.D. Effects of omega-3 and -6 polyunsaturated fatty acids on ovine follicular cell steroidogenesis, embryo development and molecular markers of fatty acid metabolism. Reproduction 2011, 141, 105–118. [Google Scholar] [CrossRef]
- Sanchez-Lazo, L.; Brisard, D.; Elis, S.; Maillard, V.; Uzbekov, R.; Labas, V.; Desmarchais, A.; Papillier, P.; Monget, P.; Uzbekova, S. Fatty acid synthesis and oxidation in cumulus cells support oocyte maturation in bovine. Mol. Endocrinol. 2014, 28, 1502–1521. [Google Scholar] [CrossRef] [PubMed]
- Nuttinck, F.; Gall, L.; Ruffini, S.; Laffont, L.; Clement, L.; Reinaud, P.; Adenot, P.; Grimard, B.; Charpigny, G.; Marquant-Le Guienne, B. Ptgs2-related pge2 affects oocyte mapk phosphorylation and meiosis progression in cattle: Late effects on early embryonic development. Biol. Reprod. 2011, 84, 1248–1257. [Google Scholar] [CrossRef] [PubMed]
- Marei, W.F.; Wathes, D.C.; Fouladi-Nashta, A.A. The effect of linolenic acid on bovine oocyte maturation and development. Biol. Reprod. 2009, 81, 1064–1072. [Google Scholar] [CrossRef]
- Peiris, H.N.; Vaswani, K.; Almughlliq, F.; Koh, Y.Q.; Mitchell, M.D. Review: Eicosanoids in preterm labor and delivery: Potential roles of exosomes in eicosanoid functions. Placenta 2017, 54, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Hailay, T.; Hoelker, M.; Poirier, M.; Gebremedhn, S.; Rings, F.; Saeed-Zidane, M.; Salilew-Wondim, D.; Dauben, C.; Tholen, E.; Neuhoff, C.; et al. Extracellular vesicle-coupled mirna profiles in follicular fluid of cows with divergent post-calving metabolic status. Sci. Rep. 2019, 9, 12851. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, C. Exosome-mediated communication in the ovarian follicle. J. Assist. Reprod. Genet. 2016, 33, 303–311. [Google Scholar] [CrossRef]
- da Silveira, J.C.; Andrade, G.M.; Del Collado, M.; Sampaio, R.V.; Sangalli, J.R.; Silva, L.A.; Pinaffi, F.V.L.; Jardim, I.B.; Cesar, M.C.; Nogueira, M.F.G.; et al. Supplementation with small-extracellular vesicles from ovarian follicular fluid during in vitro production modulates bovine embryo development. PLoS ONE 2017, 12, e0179451. [Google Scholar] [CrossRef]
- Machtinger, R.; Laurent, L.C.; Baccarelli, A.A. Extracellular vesicles: Roles in gamete maturation, fertilization and embryo implantation. Hum. Reprod. Update 2016, 22, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Singina, G.; Shedova, E.; Uzbekov, R.; Uzbekova, S. Effect of different concentrations of follicular fluid exosome-like extracellular vesicles on in vitro oocyte maturation and embryo development in cattle. In Annual Conference of the International Embryo Transfer Society IETS; Reproduction, Fertility and Development: Savannah, GA, USA, 2022; Volume 34, p. 305. [Google Scholar]
- Shaaker, M.; Rahimipour, A.; Nouri, M.; Khanaki, K.; Darabi, M.; Farzadi, L.; Shahnazi, V.; Mehdizadeh, A. Fatty acid composition of human follicular fluid phospholipids and fertilization rate in assisted reproductive techniques. Iran. Biomed. J. 2012, 16, 162–168. [Google Scholar]
- González-Serrano, A.F.; Ferreira, C.R.; Pirro, V.; Lucas-Hahn, A.; Heinzmann, J.; Hadeler, K.-G.; Baulain, U.; Aldag, P.; Meyer, U.; Piechotta, M.; et al. Effects of long-term dietary supplementation with conjugated linoleic acid on bovine oocyte lipid profile. Reprod. Fertil. Dev. 2015, 28, 1326–1339. [Google Scholar] [CrossRef]
- Marei, W.F.; Wathes, D.C.; Fouladi-Nashta, A.A. Impact of linoleic acid on bovine oocyte maturation and embryo development. Reproduction 2010, 139, 979–988. [Google Scholar] [CrossRef]
- Robker, R.L.; Wu, L.L.Y.; Yang, X. Inflammatory pathways linking obesity and ovarian dysfunction. J. Reprod. Immunol. 2011, 88, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Sohel, M.M.H.; Hoelker, M.; Noferesti, S.S.; Salilew-Wondim, D.; Tholen, E.; Looft, C.; Rings, F.; Uddin, M.J.; Spencer, T.E.; Schellander, K.; et al. Exosomal and non-exosomal transport of extra-cellular micrornas in follicular fluid: Implications for bovine oocyte developmental competence. PLoS ONE 2013, 80, e78505. [Google Scholar] [CrossRef] [PubMed]
- da Silveira, J.C.; de Avila, A.; Garrett, H.L.; Bruemmer, J.E.; Winger, Q.A.; Bouma, G.J. Cell-secreted vesicles containing micrornas as regulators of gamete maturation. J. Endocrinol. 2018, 236, R15–R27. [Google Scholar] [CrossRef] [PubMed]











| Cluster | m/z | p-Value | Lipid Ion (Carbons: Unsaturation) |
|---|---|---|---|
| 1 | m/z 794.58+ | 7.94 × 10−4 | PC(37:5)/PC(O-38:5)/PC(P-38:4)/PE(40:5) |
| 1 | m/z 808.58+ | 9.693 × 10−3 | PC(38:5)/PE(41:5)/PE(P-42:4) |
| 1 | m/z 854.56+ | 3.56 × 10−2 | PC(42:10)/PC(41:3)/PC(O-42:3)/PC(P-42:2)/PE(44:3) |
| 1 | m/z 882.59+ | 4.19 × 10−2 | PC(44:10)/PC(O-44:3) |
| 1 | m/z 613.19− | 3.10 × 10−2 | LPI(19:0)/LPI(O-20:0) |
| 2 | m/z 675.56+ | 4.33 × 10−2 | SM(d32:1) |
| 2 | m/z 689.57+ | 3.11 × 10−2 | SM(d33:1) |
| 2 | m/z 806.57+ | 4.63 × 10−2 | PC(38:6)/PE(41:6)/PE(O-42:6) |
| 2 | m/z 830.56+ | 3.27 × 10−2 | PC(40:8)/PC(39:1)/PC(O-40:1)/PC(P-40:0)/PE(42:1) |
| 2 | m/z 836.62+ | 2.59 × 10−2 | PC(40:5)/PE(44:12) |
| 3 | m/z 672.43+ | 6.06 × 10−3 | PE(31:3) |
| 3 | m/z 726.56+ | 4.05 × 10−3 | PC(32:4)/-/PE(35:4)/PE(O-36:4)/PE(P-36:3) |
| 3 | m/z 727.56+ | 1.22 × 10−2 | SM(d36:3) |
| 3 | m/z 728.53+ | 1.38 × 10−2 | PC(32:3)/PC(P-33:2)/PE(35:3)/PE(O-36:3)/PE(P-36:2) |
| 3 | m/z 326.18− | 1.50 × 10−2 | LPC(4:0)/PC(O-4:0)/LPC(O-5:0) |
| 4 | m/z 340.31− | 1.98 × 10−2 | LPC(5:0) |
| 5 | m/z 620.21+ | 2.04 × 10−2 | PE(27:1)/PE(P-28:0) |
| 5 | m/z 617.18− | 6.59 × 10−3 | LPI(20:5) |
| 5 | m/z 618.19− | 1.66 × 10−2 | PE(27:1)/PE(P-28:0) |
| 5 | m/z 631.17− | 8.66 × 10−3 | None |
| 5 | m/z 650.14− | 3.91 × 10−2 | PS(26:0)/LPS(-OMe-226:1) |
| 5 | m/z 917.26− | 4.82 × 10−2 | PI(40:2) |
| 6 | m/z 730.54+ | 3.89 × 10−2 | PC(32:2)/PC(O-33:2)/PC(P-33:1)/PE(35:2)/PE(P-36:1) |
| 6 | m/z 733.57+ | 4.14 × 10−2 | SM(d36:0) |
| 6 | m/z 790.63+ | 1.61 × 10−3 | PC(37:7)/PC(P-38:6)/PC(36:0)/PE(39:0)/PE(40:7) |
| Pathways (m/z- Features) | Total | Enrichment | p-Value (Fisher) |
|---|---|---|---|
| Glycosylphosphatidylinositol (GPI)-anchor biosynthesis | 6 | 83.4 | 0.040 |
| Glycerophospholipid metabolism | 156 | 6.4 | 0.012 |
| Cytochrome P450 metabolism | 53 | 9.4 | 0.29 |
| Vitamin B9 (folate) metabolism | 33 | 15.2 | 0.17 |
| Vitamin A (retinol) metabolism | 67 | 7.5 | 0.28 |
| Phosphatidylinositol phosphate metabolism | 59 | 8.5 | 0.12 |
| Carbon fixation | 10 | 8.3 | 0.65 |
| Galactose metabolism | 41 | 4.1 | 0.16 |
| Putative anti-inflammatory metabolites formation from EPA (C20:5) | 27 | 3.1 | 0.96 |
| Fatty acid oxidation (peroxisome) | 28 | 3.0 | 0.40 |
| Fructose and mannose metabolism | 33 | 2.5 | 0.88 |
| Fatty acid oxidation | 35 | 2.4 | 0.40 |
| Pentose phosphate pathway | 37 | 2.3 | 0.40 |
| Omega-3 fatty acid metabolism | 39 | 2.1 | 0.65 |
| Glycolysis and Gluconeogenesis | 49 | 1.7 | 0.99 |
| Sialic acid metabolism | 107 | 1.6 | 0.53 |
| Squalene and cholesterol biosynthesis | 55 | 1.5 | 0.79 |
| Omega-6 fatty acid metabolism | 55 | 1.5 | 0.40 |
| Phosphatidylinositol phosphate metabolism | 59 | 1.4 | 0.96 |
| Carnitine shuttle | 72 | 1.2 | 0.65 |
| Fatty acid activation | 74 | 1.1 | 0.40 |
| Prostaglandin formation from arachidonate | 78 | 1.1 | 0.40 |
| Lipids More Abundant in Exo | p-Value | Ratio Exo/MVs | Lipid Ion (Carbons: Unsaturation) |
|---|---|---|---|
| m/z 136.13+ | 4.60 × 10−2 | 2.46 | - |
| m/z 144.13− | 1.47 × 10−2 | 4.83 | - |
| m/z 188.13− | 5.68 × 10−3 | 3.59 | - |
| m/z 246.11− | 9.00 × 10−3 | 6.39 | - |
| m/z 362.15− | 1.34 × 10−2 | 2.99 | LPC(4:0)/PC(O-4:0) |
| m/z 616.48− | 9.49 × 10−3 | 2.47 | LPS(22:0) |
| m/z 675.55+ | 1.85 × 10−2 | 2.00 | SM(d32:1)/CE(18:0) |
| m/z 687.54− | 6.10 × 10−3 | 2.32 | SM(d33:1)/LPI(22:2) |
| m/z 688.54− | 6.77 × 10−3 | 2.28 | PE(32:1) |
| m/z 689.54− | 7.42 × 10−3 | 2.22 | SM(d33:0)/LPI(22:1) |
| m/z 689.56+ | 1.12 × 10−2 | 2.03 | SM(d33:1)/CE(19:0) |
| m/z 703.58+ | 1.40 × 10−2 | 2.13 | SM(d34:1)/CE(20:0) |
| m/z 704.58+ | 1.36 × 10−2 | 2.13 | PC or PE |
| m/z 705.59+ | 1.54 × 10−2 | 2.13 | PC or PE |
| m/z 715.55− | 4.55 × 10−3 | 2.18 | SM(d35:1) |
| m/z 731.60+ | 9.35 × 10−3 | 2.23 | SM(d36:1) |
| m/z 797.64− | 1.70 × 10−3 | 2.19 | SM(d41:2) |
| m/z 798.64− | 2.51 × 10−3 | 2.07 | PE(40:2) |
| m/z 815.54− | 1.21 × 10−2 | 2.38 | SM(d42:0) or PI |
| m/z 816.54− | 1.42 × 10−2 | 2.06 | Su (d18:2/C19:1) |
| m/z 853.59− | 2.91 × 10−4 | 2.14 | PI |
| m/z 854.59− | 3.96 × 10−4 | 2.06 | PC, PE, or PS |
| m/z 925.65− | 8.91 × 10−3 | 2.09 | PI |
| m/z 963.72− | 5.21 × 10−4 | 2.08 | PI |
| m/z 104.18+ | 3.75 × 10−5 | 0.43 | Choline |
| m/z 190.86+ | 5.72 × 10−3 | 0.40 | - |
| m/z 212.08+ | 4.21 × 10−5 | 0.43 | - |
| m/z 264.17+ | 2.31 × 10−11 | 0.16 | - |
| m/z 277.02− | 1.68 × 10−2 | 0.37 | - |
| m/z 280.18+ | 7.25 × 10−4 | 0.21 | - |
| m/z 325.27− | 3.65 × 10−2 | 0.48 | - |
| m/z 381.13+ | 5.57 × 10−4 | 0.31 | - |
| m/z 393.07− | 2.86 × 10−2 | 0.28 | - |
| m/z 509.10− | 1.859 × 10−2 | 0.27 | - |
| m/z 599.34− | 3.87 × 10−2 | 0.39 | LPI(18:0) |
| m/z 660.09− | 2.76 × 10−2 | 0.30 | LPS(24:0) |
| m/z 672.42+ | 4.43 × 10−3 | 0.45 | PE/PC/Cer(d18:1/24:0) |
| Lipids More Abundant in LF | p-Value | Ratio LF/SF | Lipid Ion |
|---|---|---|---|
| (Carbons: Unsaturation) | |||
| m/z 312.27− | 6.46 × 10−4 | 2.91 | LPC(3:0)/PC(O-3:0) |
| m/z 327.44+ | 2.55 × 10−3 | 3.87 | - |
| m/z 340.30− | 2.33 × 10−3 | 3.35 | LPC(5:0) |
| m/z 358.07+ | 2.23 × 10−3 | 9.88 | - |
| m/z 393.07− | 5.03 × 10−3 | 4.44 | - |
| m/z 484.24− | 1.50 × 10−3 | 2.5 | LPS(14:0)CAR(20:3) |
| m/z 498.24− | 1.29 × 10−3 | 2.78 | LPE(20:5)//LPE(17:2) |
| m/z 509.10− | 2.51 × 10−3 | 4.7 | - |
| m/z 660.09− | 2.91 × 10−4 | 6.16 | LPS(22:0) |
| m/z 672.42+ | 2.15 × 10−3 | 2.25 | Cer(d18:1/24:0) |
| m/z 526.33− | 1.87 × 10−6 | 0.18 | LPE(22:5) |
| m/z 528.33− | 1.80 × 10−7 | 0.28 | LPE(22:4) |
| m/z 597.32− | 5.20 × 10−3 | 0.22 | PS(41a:7) |
| m/z 599.34− | 7.05 × 10−4 | 0.16 | LPI(18:0) |
| m/z 675.55+ | 1.30 × 10−3 | 0.36 | CE(17:1) |
| m/z 701.55+ | 2.12 × 10−3 | 0.45 | CE(20:1) |
| m/z 706.54+ | 1.69 × 10−5 | 0.3 | PE(32:4) |
| m/z 722.50− | 5.06 × 10−6 | 0.26 | PE 34a:0 |
| m/z 731.60+ | 5.15 × 10−4 | 0.31 | SM(d36:1) |
| m/z 734.57+ | 1.11 × 10−5 | 0.25 | PC(32:0) |
| m/z 748.52− | 1.15 × 10−5 | 0.27 | PE(P-38:5)/PE(O-38:6) |
| m/z 749.51− | 4.04 × 10−6 | 0.27 | PI(28:2) |
| m/z 750.53− | 1.30 × 10−5 | 0.28 | PE(P-38:4) |
| m/z 757.55+ | 3.66 × 10−13 | 0.29 | SM(d38:2) |
| m/z 760.50− | 2.90 × 10−5 | 0.27 | PE(38:7) |
| m/z 768.53− | 1.20 × 10−7 | 0.29 | PE(38a:4) |
| m/z 788.53− | 2.02 × 10−5 | 0.3 | PE(40:7) |
| m/z 790.53− | 1.91 × 10−6 | 0.32 | PE(40:6) |
| m/z 794.54− | 9.43 × 10−8 | 0.31 | PS(P-38:4) |
| m/z 813.68+ | 8.16 × 10−6 | 0.25 | SM(d42:2) |
| m/z 815.69+ | 1.19 × 10−6 | 0.26 | SM(d42:1) |
| Pathways | Total | Hits | Enrichment | Raw p-Value | Impact |
|---|---|---|---|---|---|
| Glycerophospholipid metabolism | 36 | 3 | 25.8 | 1.12 × 10−4 | 0.218 |
| Glycosylphosphatidylinositol (GPI)-anchor biosynthesis | 14 | 2 | 44.3 | 7.46 × 10−4 | 0.004 |
| Sphingolipid metabolism | 21 | 2 | 29.5 | 1.71 × 10−3 | 0.269 |
| Linoleic acid metabolism | 5 | 1 | 62.0 | 1.60 × 10−2 | 0 |
| Alpha-linolenic acid metabolism | 13 | 1 | 23.8 | 4.13 × 10−2 | 0 |
| Phosphatidylinositol signaling system | 28 | 1 | 11.1 | 8.72 × 10−2 | 0.097 |
| Inositol phosphate metabolism | 30 | 1 | 10.3 | 9.32 × 10−2 | 0.078 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maugrion, E.; Shedova, E.N.; Uzbekov, R.; Teixeira-Gomes, A.-P.; Labas, V.; Tomas, D.; Banliat, C.; Singina, G.N.; Uzbekova, S. Extracellular Vesicles Contribute to the Difference in Lipid Composition between Ovarian Follicles of Different Size Revealed by Mass Spectrometry Imaging. Metabolites 2023, 13, 1001. https://doi.org/10.3390/metabo13091001
Maugrion E, Shedova EN, Uzbekov R, Teixeira-Gomes A-P, Labas V, Tomas D, Banliat C, Singina GN, Uzbekova S. Extracellular Vesicles Contribute to the Difference in Lipid Composition between Ovarian Follicles of Different Size Revealed by Mass Spectrometry Imaging. Metabolites. 2023; 13(9):1001. https://doi.org/10.3390/metabo13091001
Chicago/Turabian StyleMaugrion, Emilie, Ekaterina N. Shedova, Rustem Uzbekov, Ana-Paula Teixeira-Gomes, Valerie Labas, Daniel Tomas, Charles Banliat, Galina N. Singina, and Svetlana Uzbekova. 2023. "Extracellular Vesicles Contribute to the Difference in Lipid Composition between Ovarian Follicles of Different Size Revealed by Mass Spectrometry Imaging" Metabolites 13, no. 9: 1001. https://doi.org/10.3390/metabo13091001
APA StyleMaugrion, E., Shedova, E. N., Uzbekov, R., Teixeira-Gomes, A. -P., Labas, V., Tomas, D., Banliat, C., Singina, G. N., & Uzbekova, S. (2023). Extracellular Vesicles Contribute to the Difference in Lipid Composition between Ovarian Follicles of Different Size Revealed by Mass Spectrometry Imaging. Metabolites, 13(9), 1001. https://doi.org/10.3390/metabo13091001